Key topics in orthopaedic oncology are discussed in the new JBJS Guest Editorial What’s New in Musculoskeletal Tumor Surgery. Here, we highlight studies of high importance, as selected by coauthor Michelle Ghert, MD, FRCSC.
Specific Soft-Tissue Sarcoma Subtypes
A multicenter phase-2 study assessed the effectiveness of atezolizumab, an immune checkpoint inhibitor, in treating advanced alveolar soft-part sarcoma in 52 adult and pediatric patients. Approximately one-third of the cohort demonstrated a sustained response to atezolizumab1. “This study highlights the potential of immunotherapy in managing rare and difficult-to-treat sarcomas, underlining the importance of further research,” write Guest Editorial authors Drs. Ghert and Gazendam.
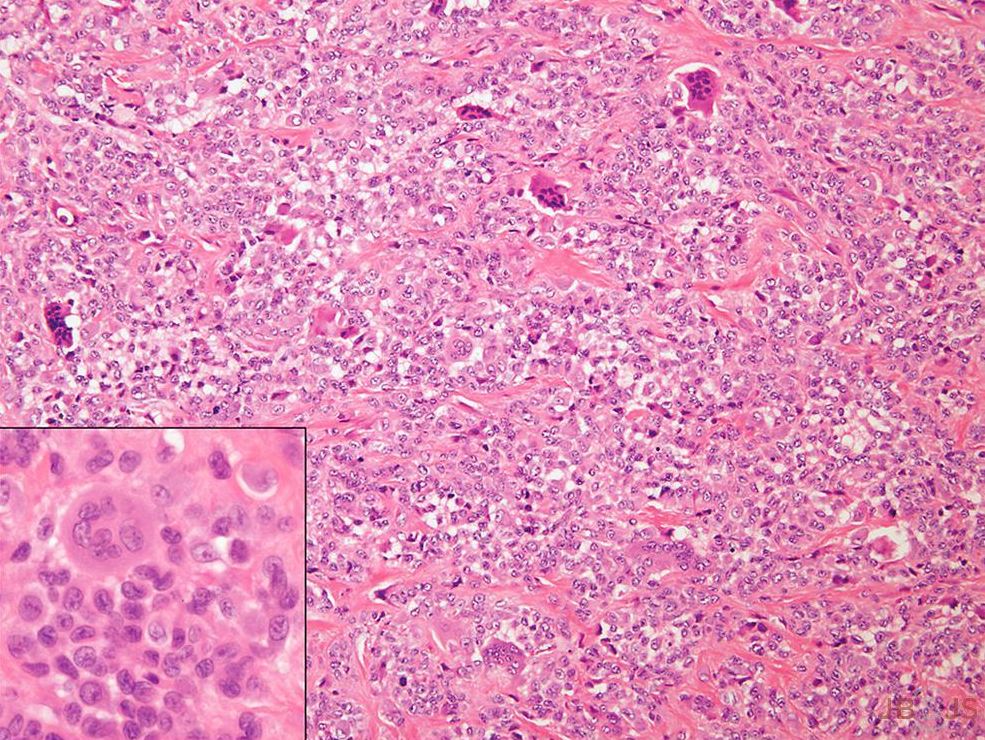
Tenosynovial Giant Cell Tumor
The efficacy of vimseltinib, a colony-stimulating factor 1 (CSF-1) inhibitor, in treating tenosynovial giant cell tumor was assessed in the MOTION trial, a phase-3 study that included 123 patients. The objective response rate in the vimseltinib group was significantly higher than that in the placebo group (40% vs. 0%; p < 0.0001). The study authors noted that vimseltinib produced “clinically meaningful functional and symptomatic improvement . . . providing an effective treatment option for these patients.”2
International Collaboration, Consensus Work, and Data-Sharing
The Birmingham Orthopaedic Oncology Consensus Meeting, held in January 2024, aimed to produce consensus statements and to foster international collaboration among >300 delegates from >50 countries. The meeting facilitated debate regarding controversies in orthopaedic oncology, including the prevention and management of surgical site infection in bone tumor reconstruction and the diagnosis and management of chondrosarcoma. The resultant paper outlines some of these discussions3.
Surgical Management
A clinical practice guideline for the management of metastatic humeral disease was developed by the Musculoskeletal Tumor Society in collaboration with the American Academy of Orthopaedic Surgeons. The guideline provides 7 action statements for health-care professionals and draws attention to gaps in the literature. According to the guideline, the addition of cement may provide improved pain relief and functional mobility in the short term. For reconstruction of the proximal humerus, reverse total shoulder arthroplasty was recommended in order to reduce shoulder instability and to improve range of motion4.
PARITY Trial Secondary Analyses
The Prophylactic Antibiotic Regimens in Tumor Surgery (PARITY) Trial investigated the impact of postoperative antibiotic regimen on the rate of surgical site infection following endoprosthetic reconstruction for lower-extremity bone tumors5. Several secondary analyses were conducted, on topics ranging from thromboembolism risk factors to opioid consumption patterns in the PARITY cohort. A selection of the secondary analyses were published as a JBJS supplement. Additional details are provided in the OrthoBuzz post Now Available: JBJS Supplement on the Secondary Analyses from the PARITY Trial.
What’s New in Musculoskeletal Tumor Surgery is freely available at JBJS.org.
What’s New by Subspecialty
Each month, JBJS publishes a review of the most pertinent studies from the orthopaedic literature in a select subspecialty. To read the reports, visit the What’s New by Subspecialty collection at JBJS.org.
Recent OrthoBuzz posts include: What’s New in Musculoskeletal Basic Science, What’s New in Orthopaedic Rehabilitation, and What’s New in Shoulder and Elbow Surgery.
References
- Chen AP, Sharon E, O’Sullivan-Coyne G, Moore N, Foster JC, Hu JS, Van Tine BA, Conley AP, Read WL, Riedel RF, Burgess MA, Glod J, Davis EJ, Merriam P, Naqash AR, Fino KK, Miller BL, Wilsker DF, Begum A, Ferry-Galow KV, Deshpande HA, Schwartz GK, Ladle BH, Okuno SH, Beck JC, Chen JL, Takebe N, Fogli LK, Rosenberger CL, Parchment RE, Doroshow JH. Atezolizumab for advanced alveolar soft part sarcoma. N Engl J Med. 2023 Sep 7;389(10):911-21.
- Gelderblom H, Bhadri V, Stacchiotti S, Bauer S, Wagner AJ, van de Sande M, Bernthal NM, López Pousa A, Razak AA, Italiano A, Ahmed M, Le Cesne A, Tinoco G, Boye K, Martín-Broto J, Palmerini E, Tafuto S, Pratap S, Powers BC, Reichardt P, Casado Herráez A, Rutkowski P, Tait C, Zarins F, Harrow B, Sharma MG, Ruiz-Soto R, Sherman ML, Blay JY, Tap WD; MOTION investigators. Vimseltinib versus placebo for tenosynovial giant cell tumour (MOTION): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Jun 22;403(10445):2709-19.
- Jeys LM, Thorkildsen J, Kurisunkal V, Puri A, Ruggieri P, Houdek MT, Boyle RA, Ebeid W, Botello E, Morris GV, Laitinen MK, Abudu A, Ae K, Agarwal M, Ajit Singh V, Akiyama T, Albergo JI, Alexander J, Alpan B, Aoude A, Asavamongkolkul A, Aston W, Baad-Hansen T, Balach T, Benevenia J, Bergh P, Bernthal N, Binitie O, Boffano M, Bramer J, Branford White H, Brennan B, Cabrolier J, Calvo Haro JA, Campanacci DA, Cardoso R, Carey Smith R, Casales Fresnga N, Casanova JM, Ceballos O, Chan CM, Chung YG, Clara-Altamirano MA, Cribb G, Dadia S, Dammerer D, de Vaal M, Delgado Obando J, Deo S, Di Bella C, Donati DM, Endo M, Eralp L, Erol B, Evans S, Eward W, Fiorenza F, Freitas J, Funovics PT, Galli Serra M, Ghert M, Ghosh K, Gomez Mier LC, Gomez Vallejo J, Griffin A, Gulia A, Guzman M, Hardes J, Healey J, Hernandez A, Hesla A, Hongsaprabhas C, Hornicek F, Hosking K, Iwata S, Jagiello J, Johnson L, Johnston A, Joo MW, Jutte P, Kapanci B, Khan Z, Kobayashi H, Kollender Y, Koob S, Kotrych D, Le Nail LR, Legosz P, Lehner B, Leithner A, Lewis V, Lin P, Linares F, Lozano Calderon S, Mahendra A, Mahyudin F, Mascard E, Mattei JC, McCullough L, Medellin Rincon MR, Morgan-Jones R, Moriel Garcesco DJ, Mottard S, Nakayama R, Narhari P, O’Toole G, Vania O, Olivier A, Omar M, Ortiz-Cruz E, Ozger H, Ozkan K, Palmerini E, Papagelopoulos P, Parry M, Patton S, Petersen MM, Powell G, Puhaindran M, Raja A, Rajasekaran RB, Repsa L, Ropars M, Sambri A, Schubert T, Shehadeh A, Siegel G, Sommerville S, Spiguel A, Stevenson J, Sys G, Temple T, Traub F, Tsuchiya H, Valencia J, Van de Sande M, Vaz G, Velez Villa R, Vyrva O, Wafa H, Wan Faisham Numan WI, Wang E, Warnock D, Werier J, Wong KC, Norio Y, Zhaoming Y, Zainul Abidin S, Zamora T, Zumarraga JP, Abou-Nouar G, Gebert C, Randall RL. Controversies in orthopaedic oncology. Bone Joint J. 2024 May 1;106-B(5):425-9.
- Tedesco NS, Mesko N, Wodajo F; Management of Metastatic Humeral Disease Work Group; Staff of the American Academy of Orthopaedic Surgeons and the Musculoskeletal Tumor Society; Management of Metastatic Humeral Disease Work Group and Staff of the American Academy of Orthopaedic Surgeons and the Musculoskeletal Tumor Society. The Musculoskeletal Tumor Society clinical practice guideline on the management of metastatic humeral disease. J Am Acad Orthop Surg. 2024 May 15;32(10):e482-e488.
- Ghert M, Schneider P, Guyatt G, Thabane L, Vélez R, O’Shea T, Randall RL, Turcotte R, Wilson D, Wunder JS, Baptista AM, Cheng EY, Doung YC, Ferguson PC, Giglio V, Hayden J, Heels-Ansdell D, Khan SA, Sampath Kumar V, McKay P, Miller B, van de Sande M, Zumárraga JP, Bhandari M; Prophylactic Antibiotic Regimens in Tumor Surgery (PARITY) Investigators. Comparison of prophylactic intravenous antibiotic regimens after endoprosthetic reconstruction for lower extremity bone tumors: a randomized clinical trial. JAMA Oncol. 2022 Mar 1;8(3):345-53.
Image reproduced from: Williams RD, Honeycutt MW, Manci EA, Nimityongskul P. Pediatric Tenosynovial Giant Cell Tumor of the Flexor Hallucis Longus Tendon Sheath: A Case Report. JBJS Case Connect. 2020 Apr-Jun;10(2):e0519.