Several new papers representing a collaborative, multicenter effort to provide investigative insights into critical aspects of fracture management are now available in a special collection
Category: Trauma
A special digital collection featuring the most-read JBJS Trauma articles from 2023 to 2025 is now available. Use the following link to fill out a
Topics of interest in foot and ankle surgery are discussed in the new JBJS Guest Editorial What’s New in Foot and Ankle Surgery. Here, we
Notable findings in pediatric orthopaedics are presented in the JBJS Guest Editorial What’s New in Pediatric Orthopaedics. Here, we summarize the 5 most impactful studies,
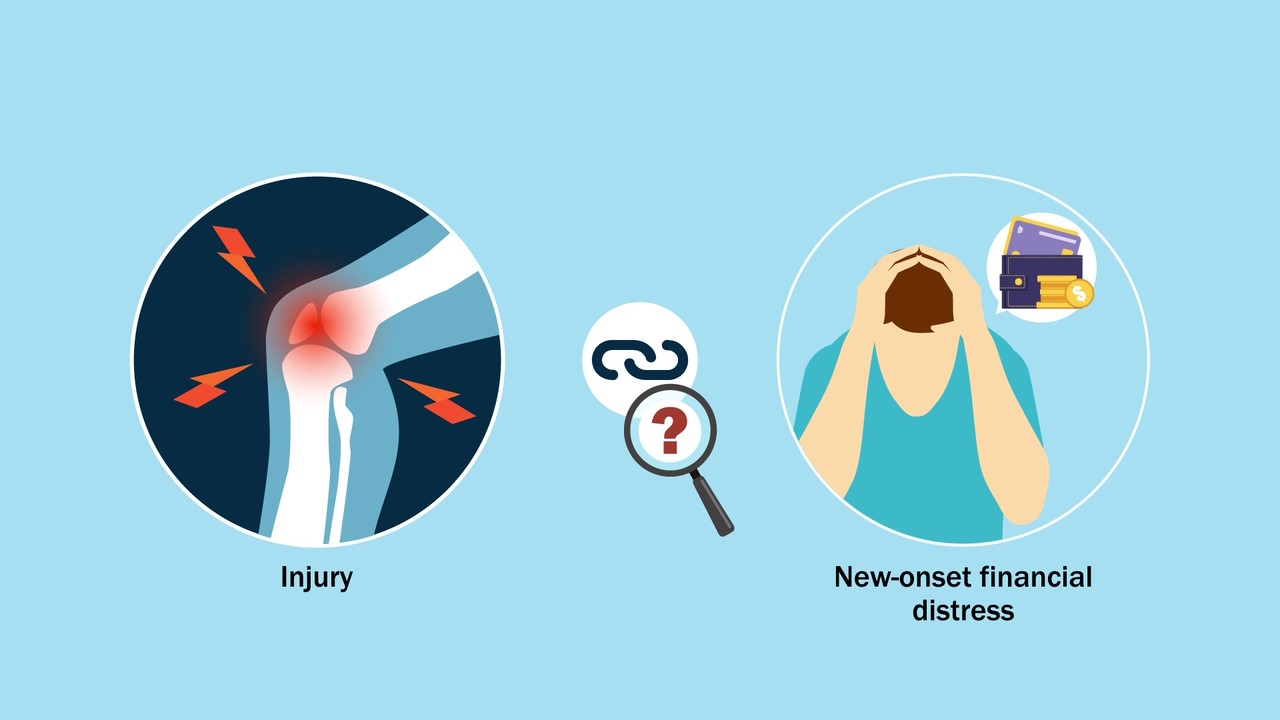
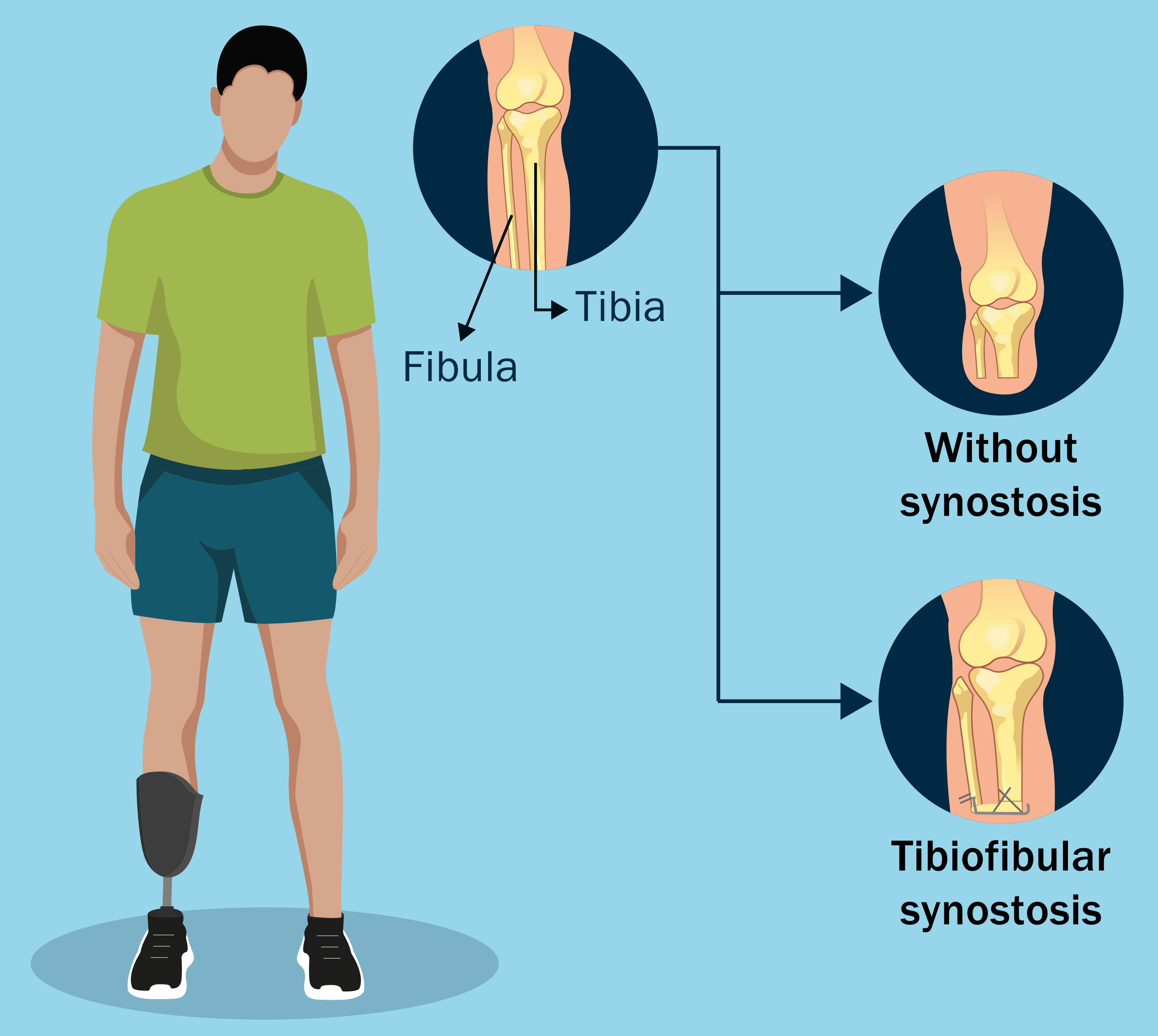
Lower-extremity injuries can have serious and wide-ranging impact on patients’ lives. What hasn’t been fully understood, note O’Hara et al. in a new study, are
Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, discusses 3 recent studies that reflect the rigorous standards, and challenges, of randomized clinical trials (RCTs).
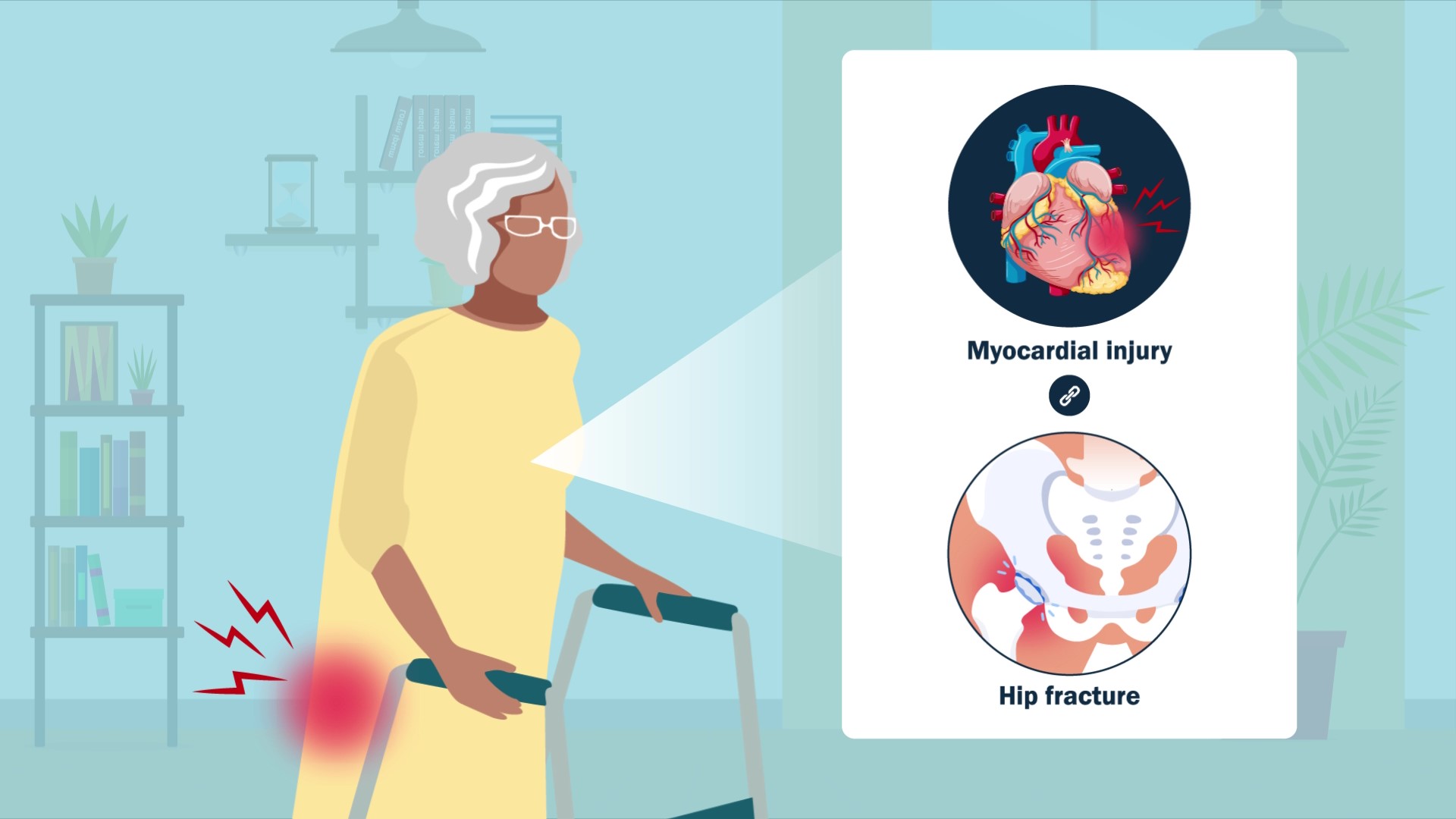
HIP ATTACK data show elevated troponin in 1 in 5 hip fracture patients, reports a new study in JBJS. For hip fracture patients with

New findings in musculoskeletal basic science are presented in the recent JBJS Guest Editorial What’s New in Musculoskeletal Basic Science. Here, we highlight the 5
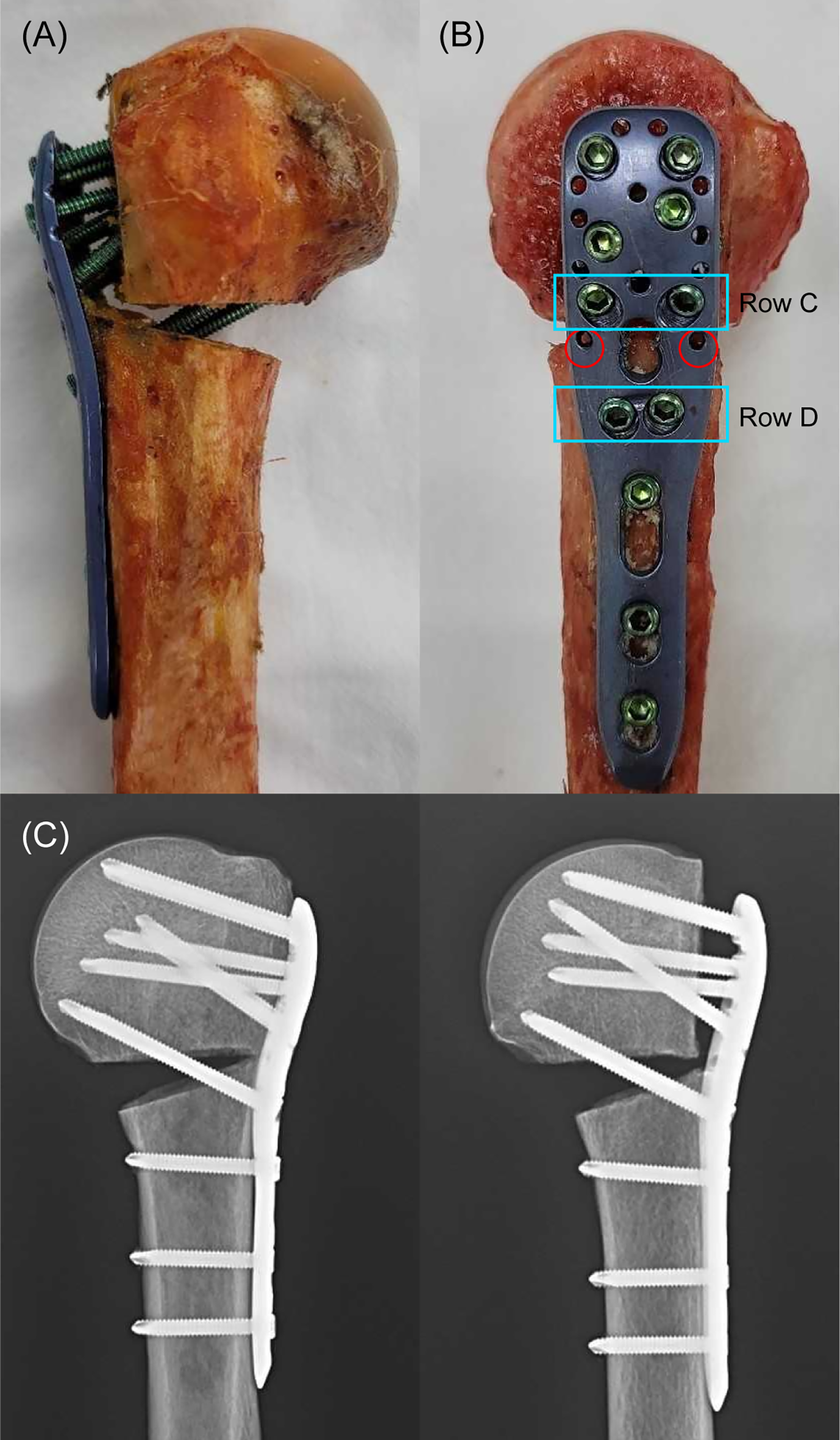
In this post, JBJS Deputy Editor for Social Media Dr. Matt Schmitz reflects on 2 recent studies of fracture fixation and the continued quest to
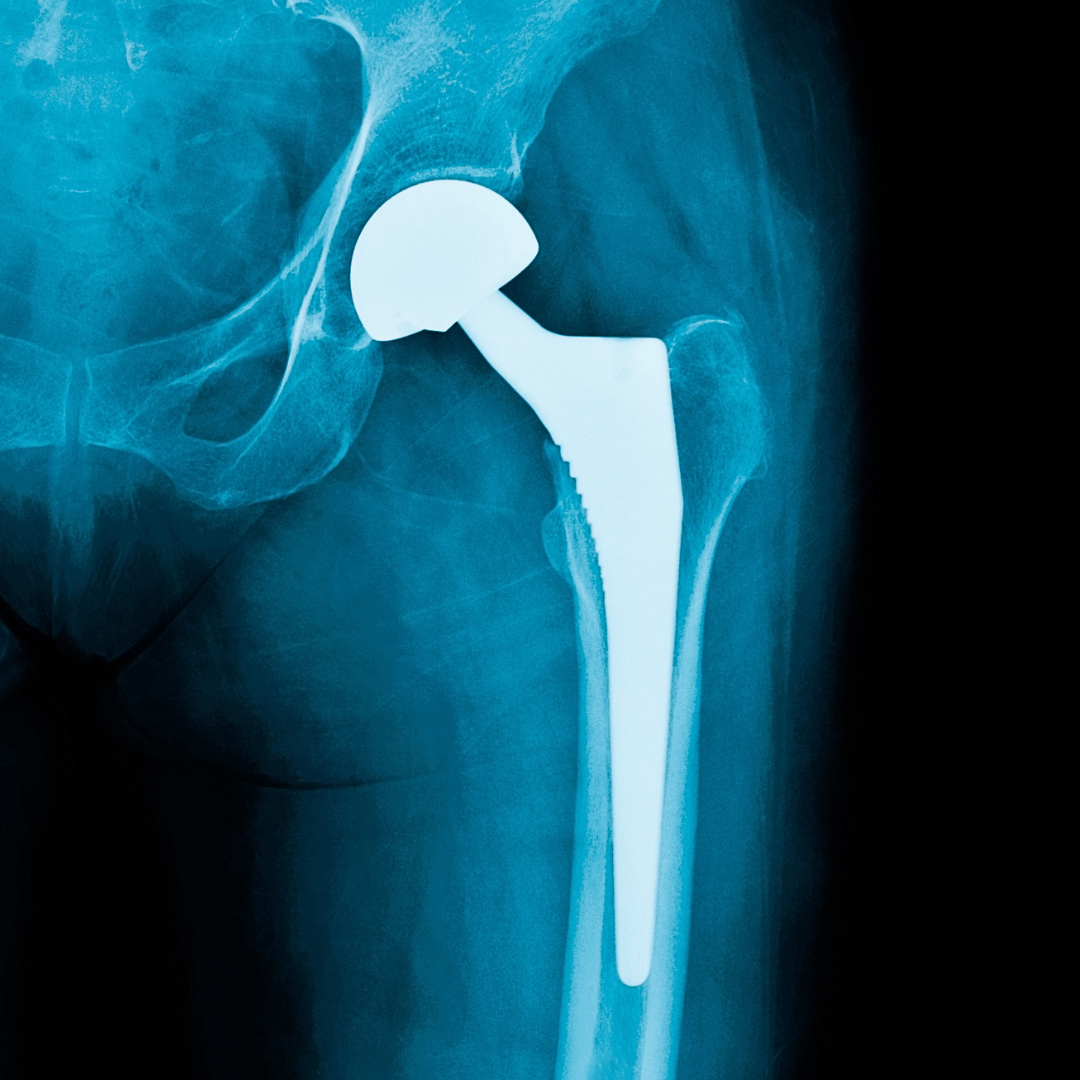
Key findings in hip surgery, including those related to fracture management and infection prevention, are presented in the new JBJS Guest Editorial What’s New in

