Several new papers representing a collaborative, multicenter effort to provide investigative insights into critical aspects of fracture management are now available in a special collection
Category: Infection
Notable findings in pediatric orthopaedics are presented in the JBJS Guest Editorial What’s New in Pediatric Orthopaedics. Here, we summarize the 5 most impactful studies,
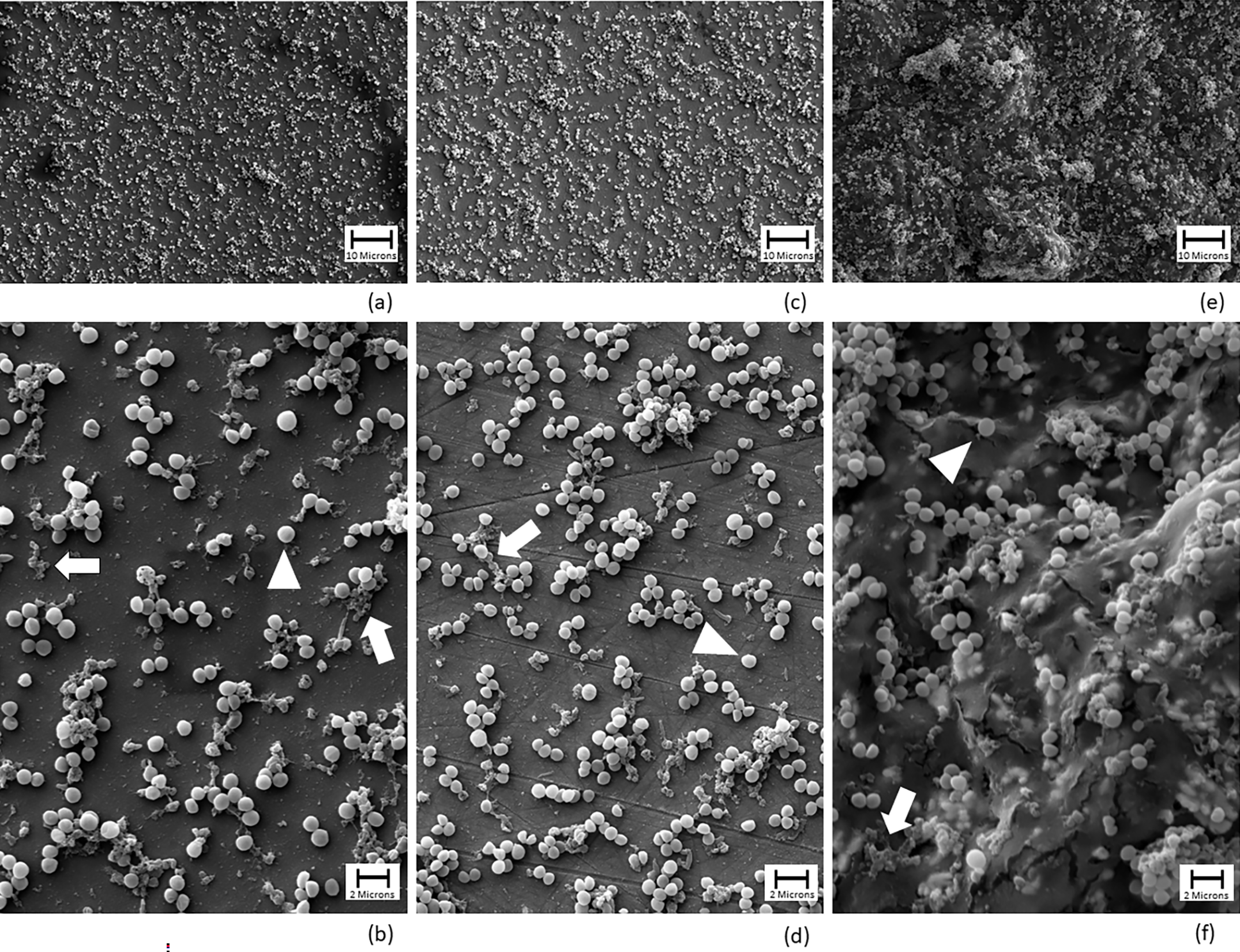
Antiseptic solutions are often used in total joint arthroplasty to help prevent or treat the challenging complication of periprosthetic joint infection. In a study now
Topics of interest in adult reconstructive knee surgery are presented in the new JBJS Guest Editorial What’s New in Adult Reconstructive Knee Surgery. Here, we
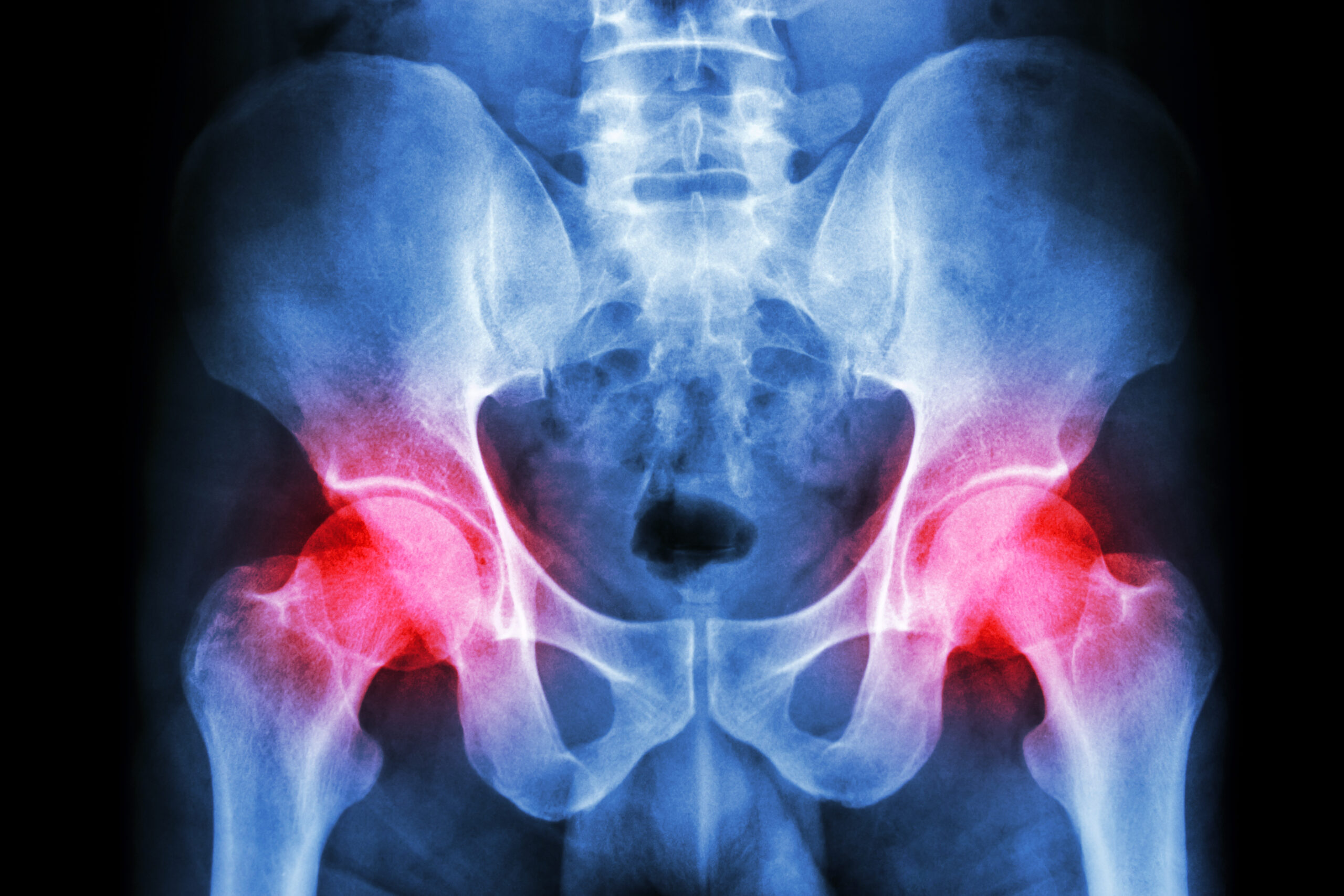
Key findings in hip surgery, including those related to fracture management and infection prevention, are presented in the new JBJS Guest Editorial What’s New in
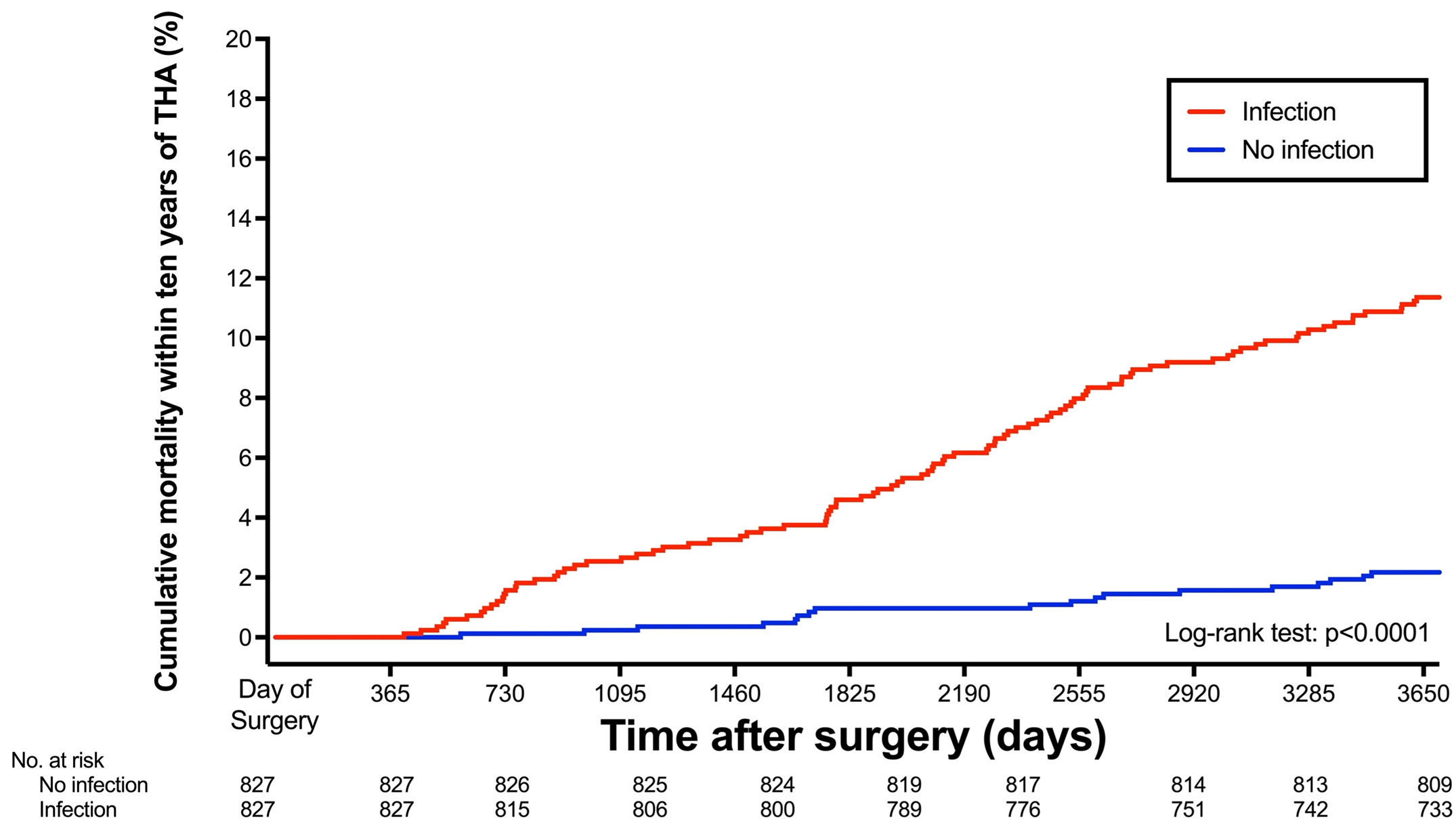
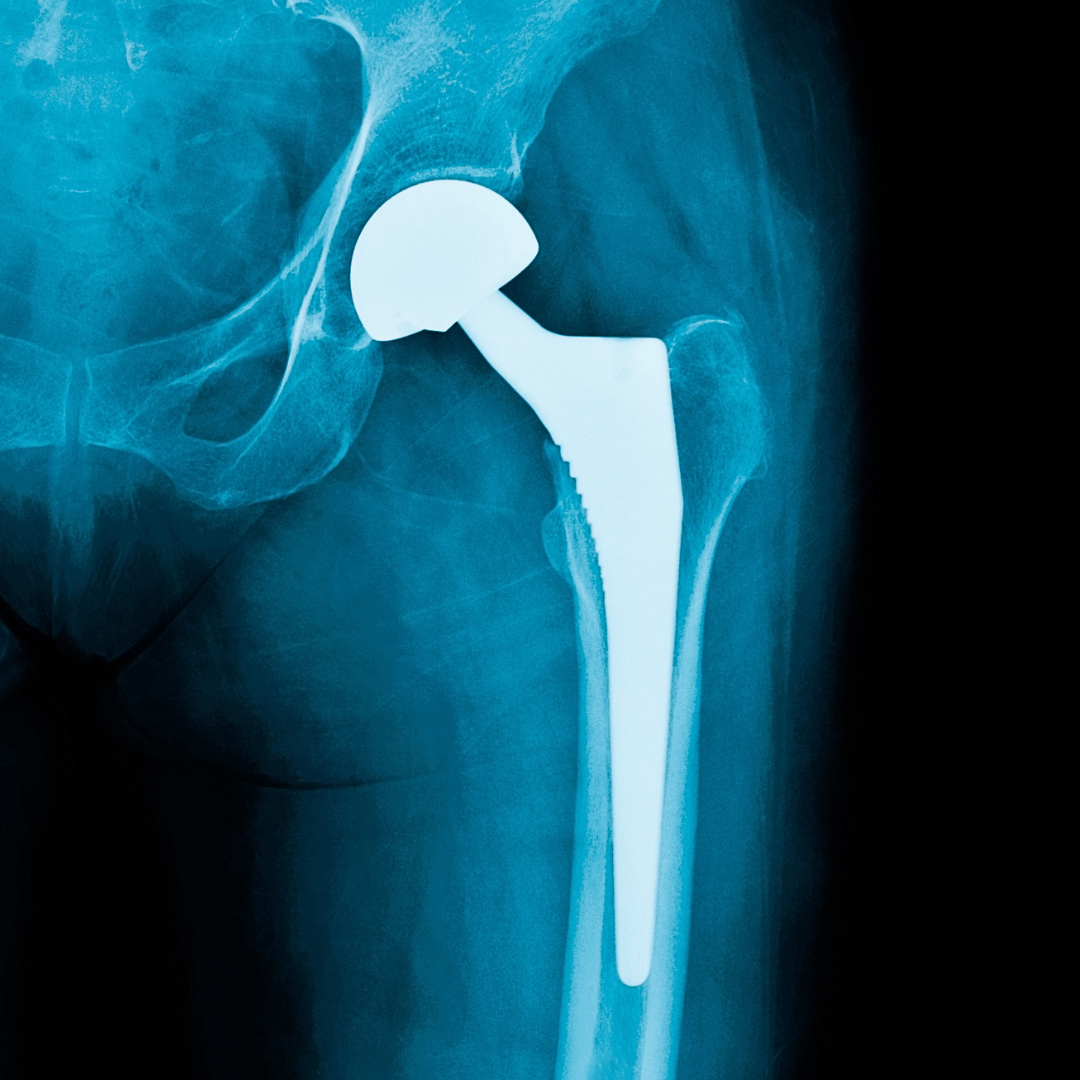
New study reports fivefold increase in 10-year risk of death after periprosthetic joint infection. Patients who develop periprosthetic joint infection (PJI) after hip replacement surgery
Key findings related to the prevention and treatment of periprosthetic joint infection (PJI), among other important topics, are presented in the new JBJS Guest Editorial
A video abstract is available with the new report in JBJS by the Major Extremity Trauma Research Consortium (METRC): The Bioburden Associated with Severe Open
A video abstract is available with the new study by Chan et al. in JBJS: A Rapid MRI Protocol for the Evaluation of Acute Pediatric Musculoskeletal
Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, discusses a new study on the impact of robotic assistance and computer navigation on periprosthetic joint

