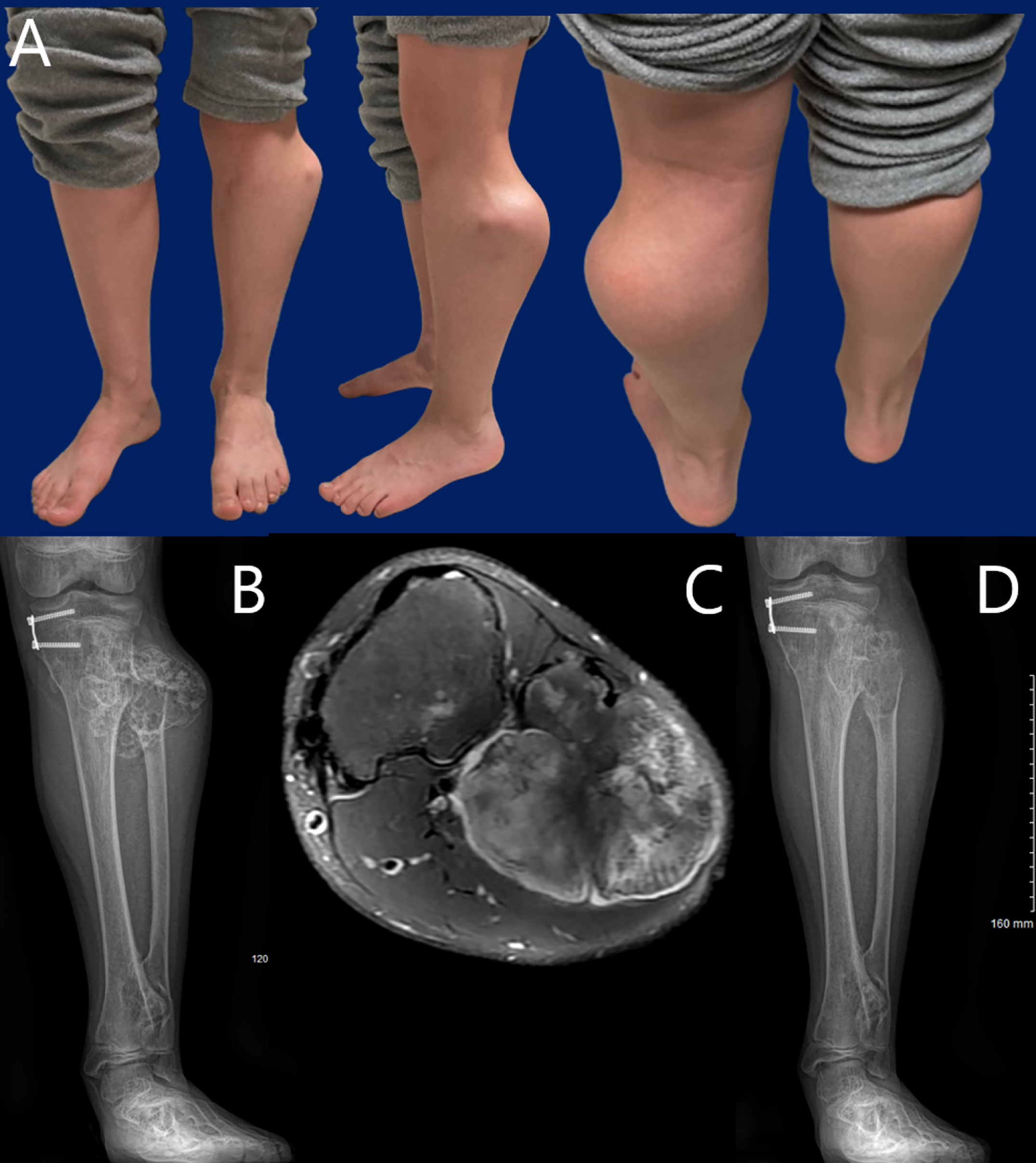
JBJS Deputy Editor for Social Media Dr. Matt Schmitz offers this post on a new study that evaluates peroneal nerve decompression and proximal fibular osteochondroma
Category: Editor’s Choice
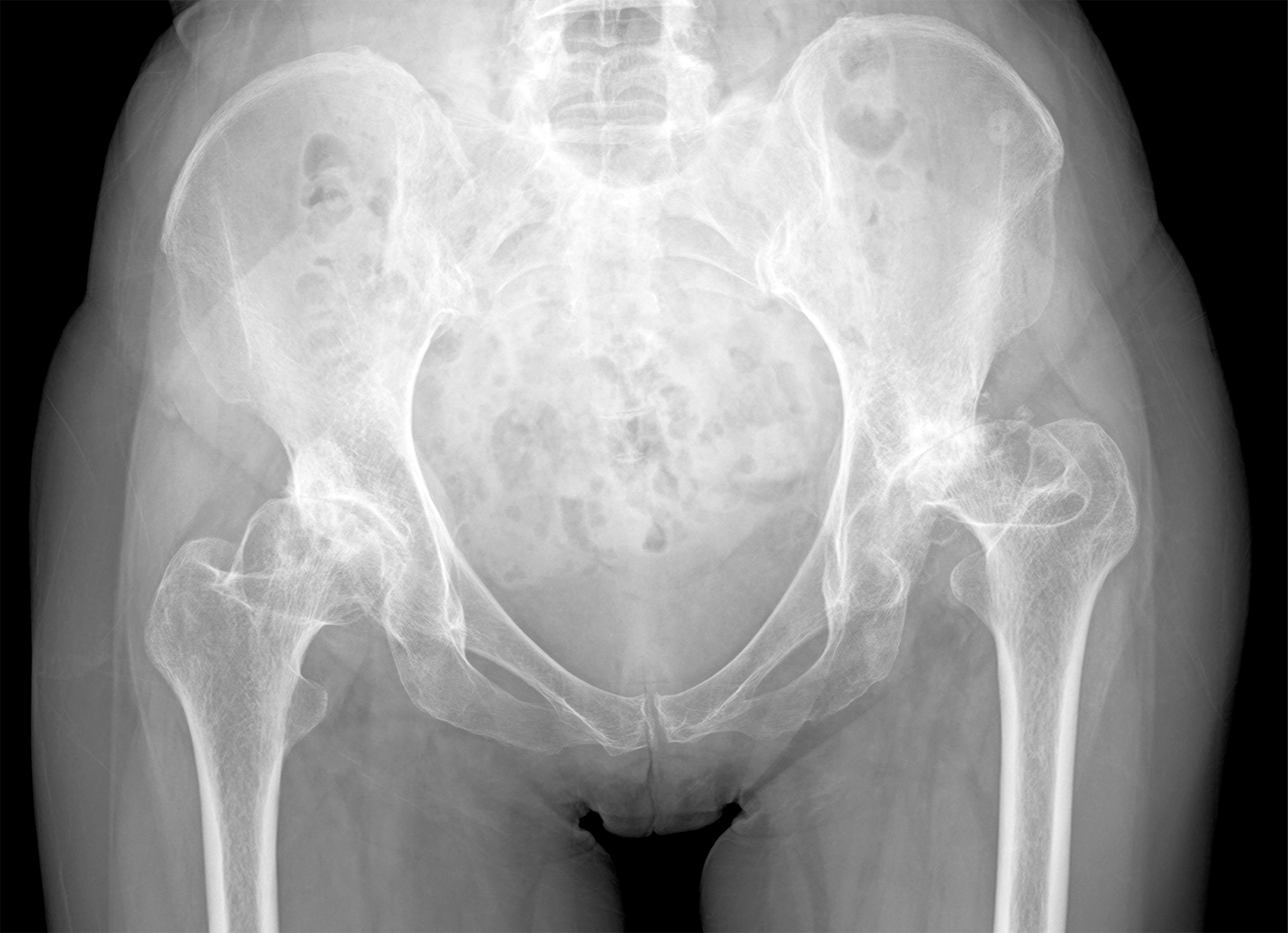
In this post, Deputy Editor for Social Media Matt Schmitz discusses a study by Sato et al. now available in JBJS. Developmental dysplasia of the
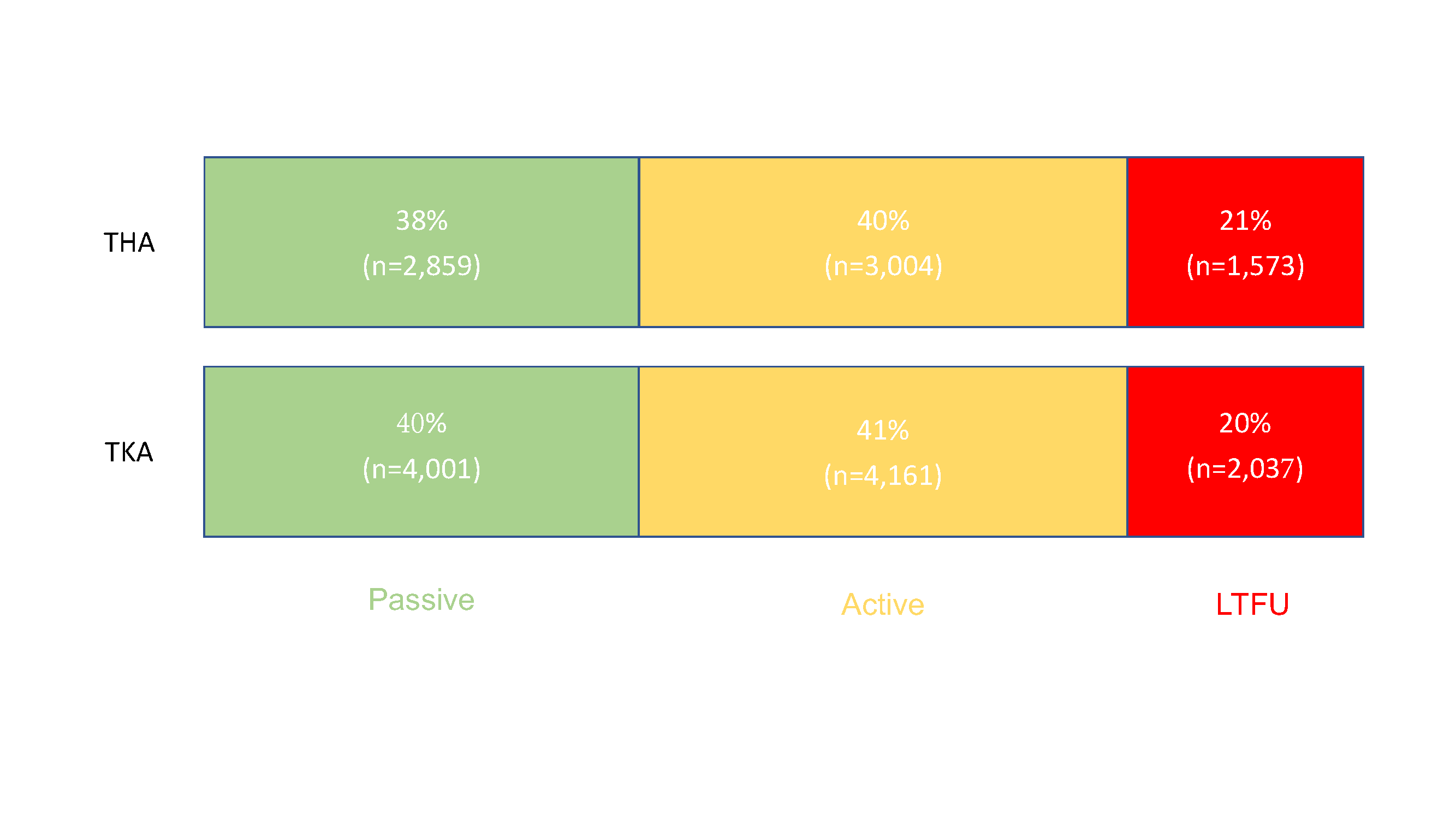
Editor-in-Chief Dr. Marc Swiontkowski reflects on a new study that examines the capture of patient-reported outcome measures (PROMs) after total joint arthroplasty. He offers a
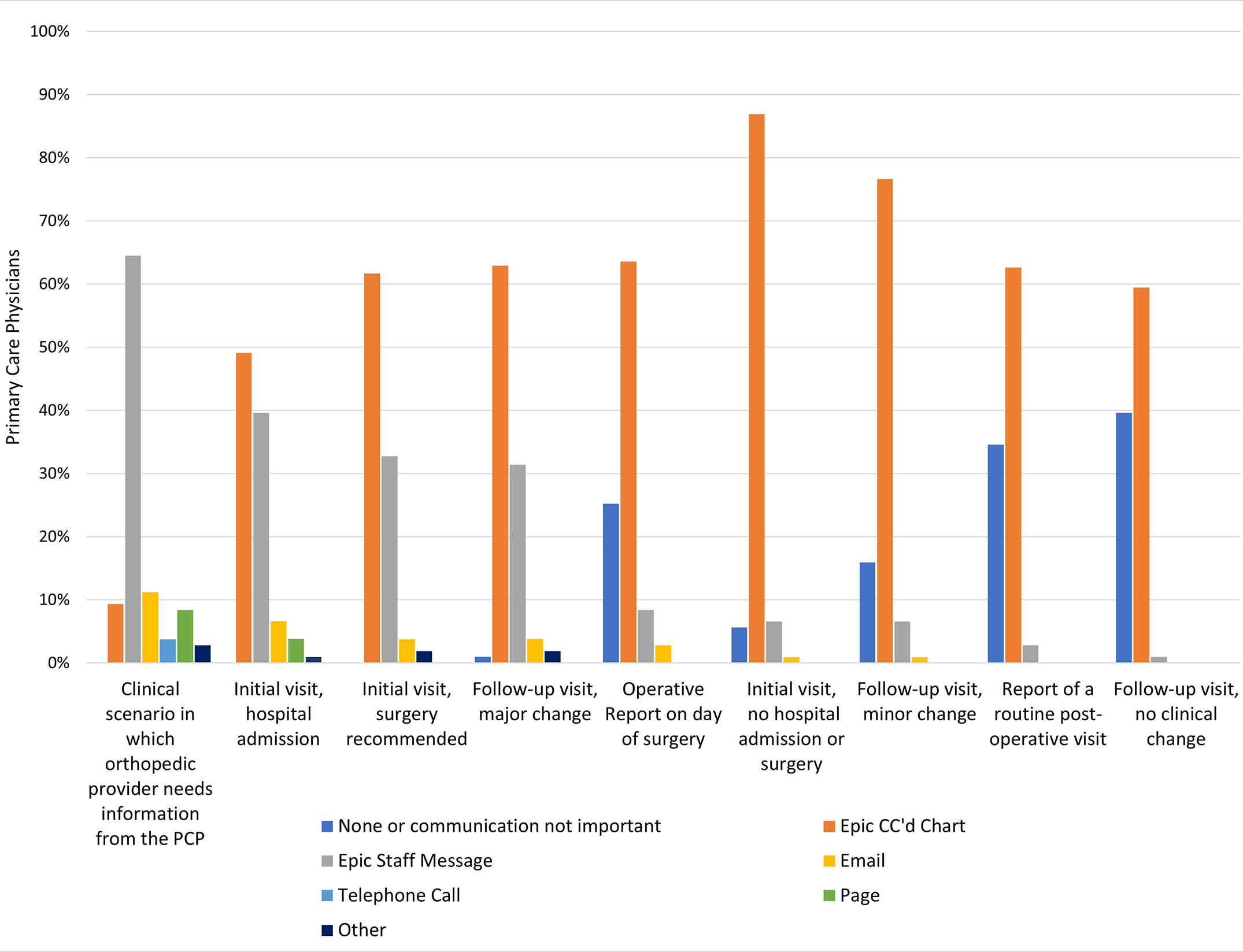
When and how would primary care physicians (PCPs) like to hear from orthopaedic surgeons regarding mutual patients? JBJS Deputy Editor for Social Media Matt Schmitz
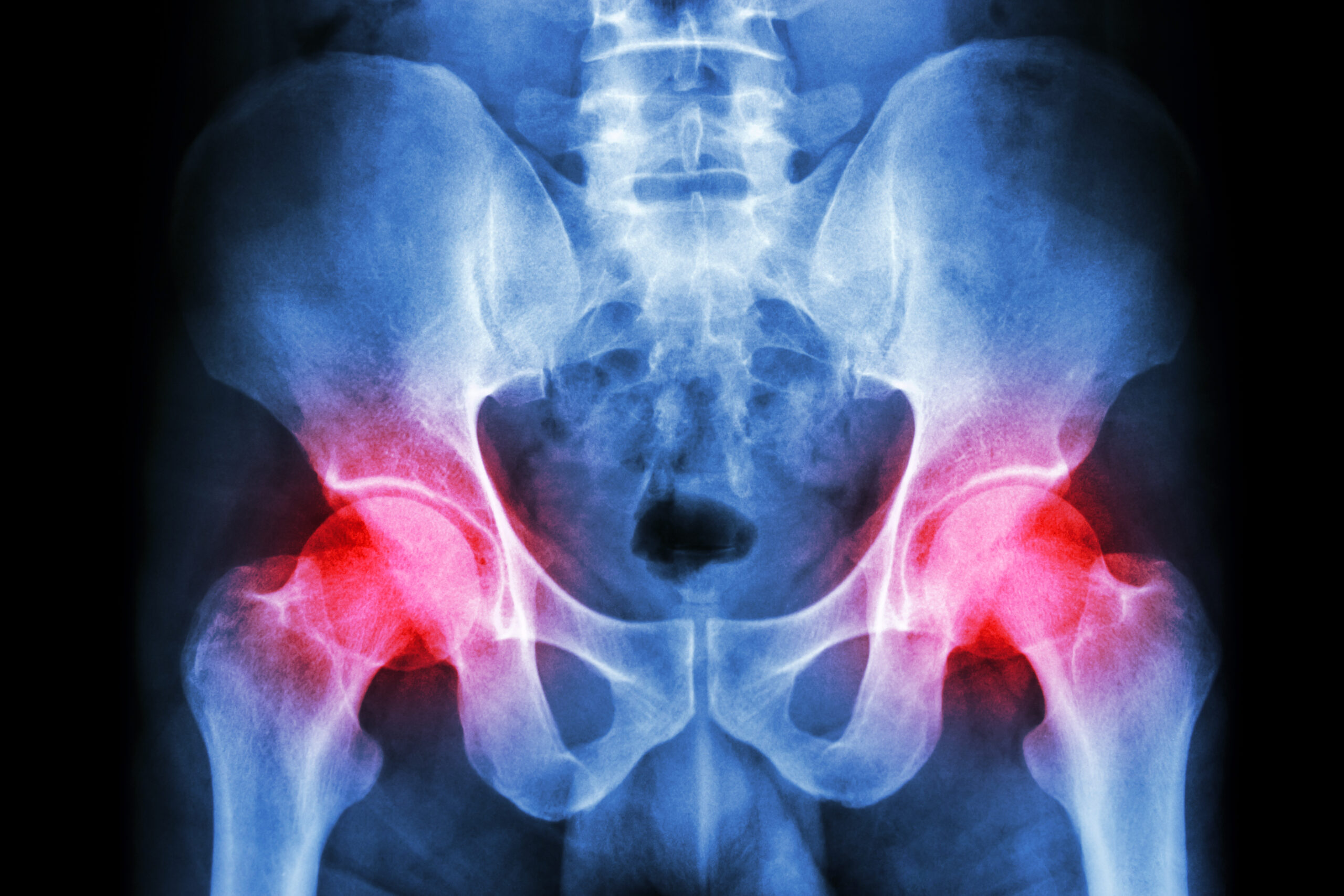
Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, discusses a new study on the impact of robotic assistance and computer navigation on periprosthetic joint
JBJS Editor-in-Chief Dr. Marc Swiontkowski shares his thoughts on a new study showing the potential of preoperative bladder scanning to predict postoperative urinary retention in
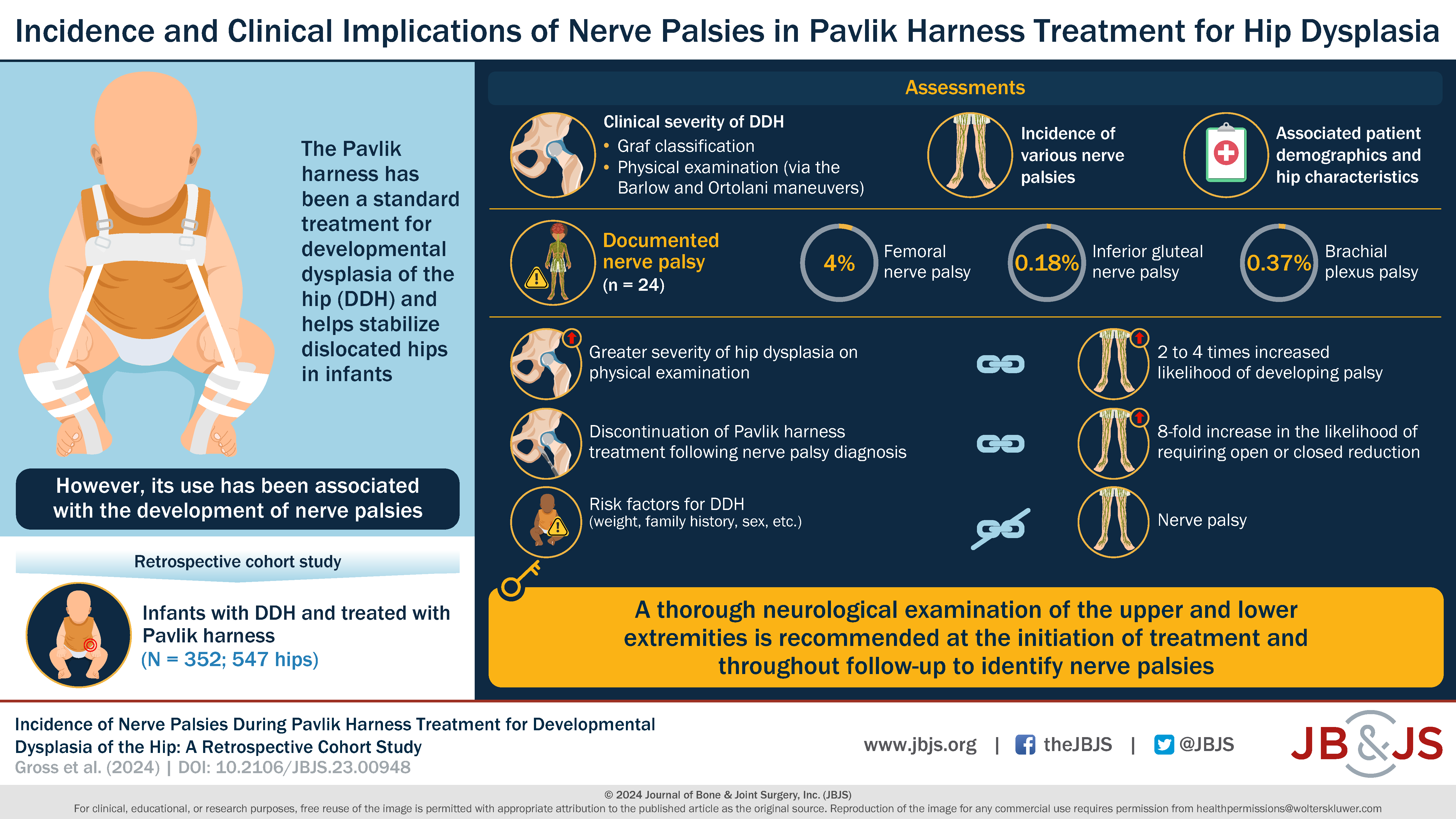
What is the incidence of nerve palsy in infants undergoing Pavlik harness treatment for developmental dysplasia of the hip (DDH)? Dr. Matt Schmitz, JBJS Deputy
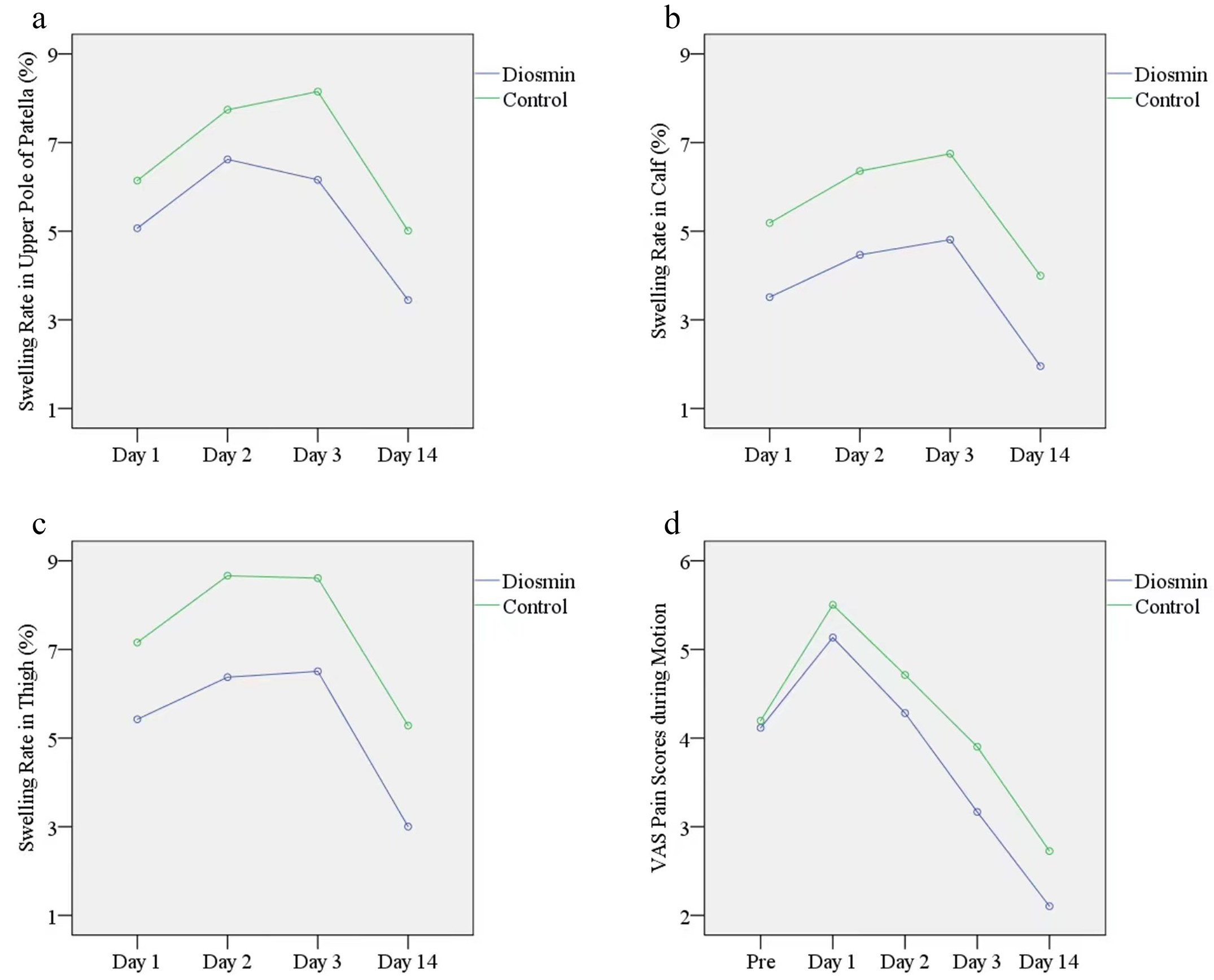
Editor-in-Chief Dr. Marc Swiontkowski offers this post on a new Level I study in JBJS evaluating the efficacy of diosmin in reducing swelling after total
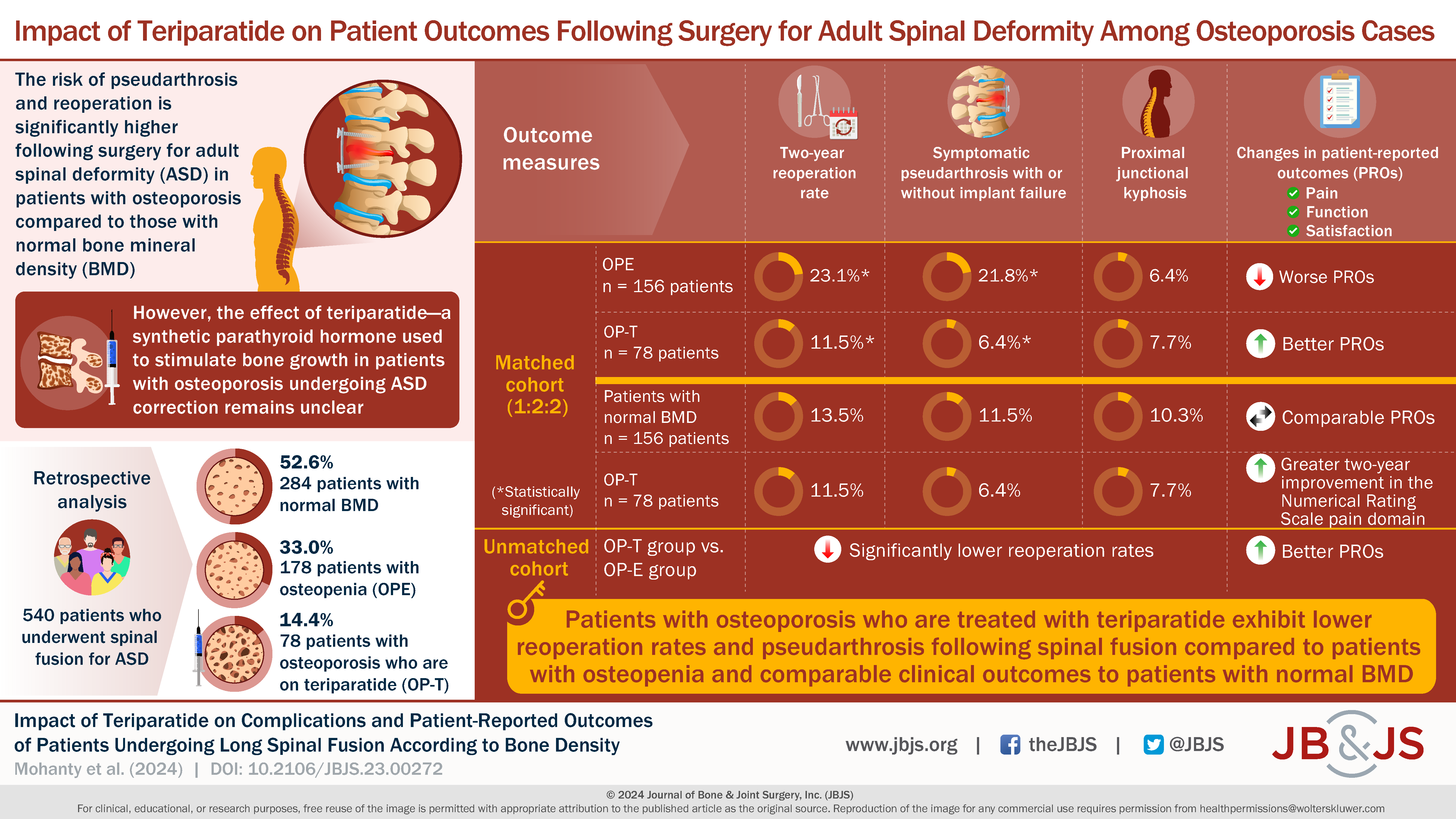
In this post, Editor-in-Chief Dr. Marc Swiontkowski discusses a new study in JBJS that assesses the impact of teriparatide use in patients with osteoporosis undergoing
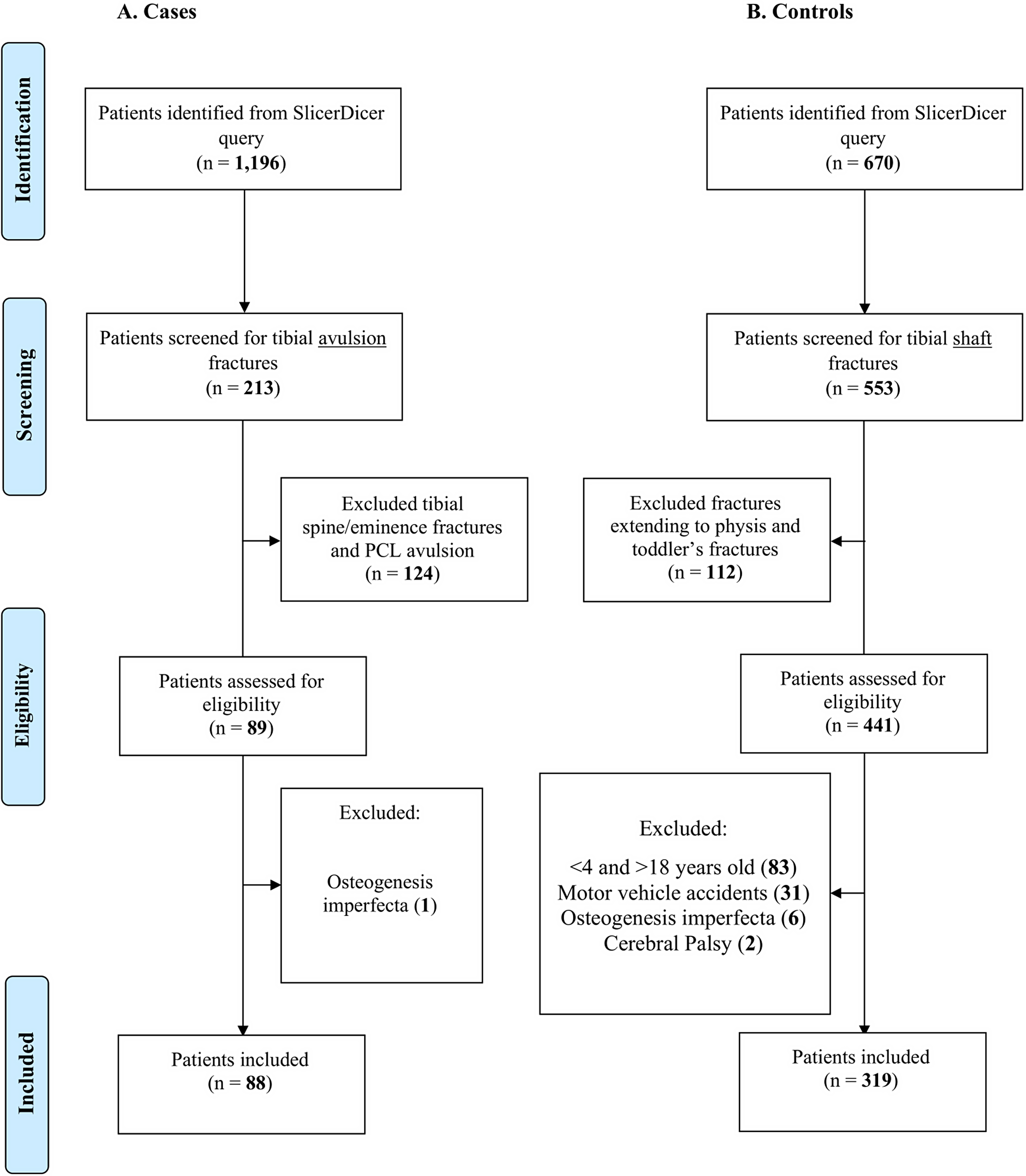
Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, offers this post on a new study that explores whether a greater proportion of pediatric patients