New findings in spine surgery are covered in the latest JBJS Guest Editorial What’s New in Spine Surgery. Here, we summarize the 5 most insightful
Category: Basic Science
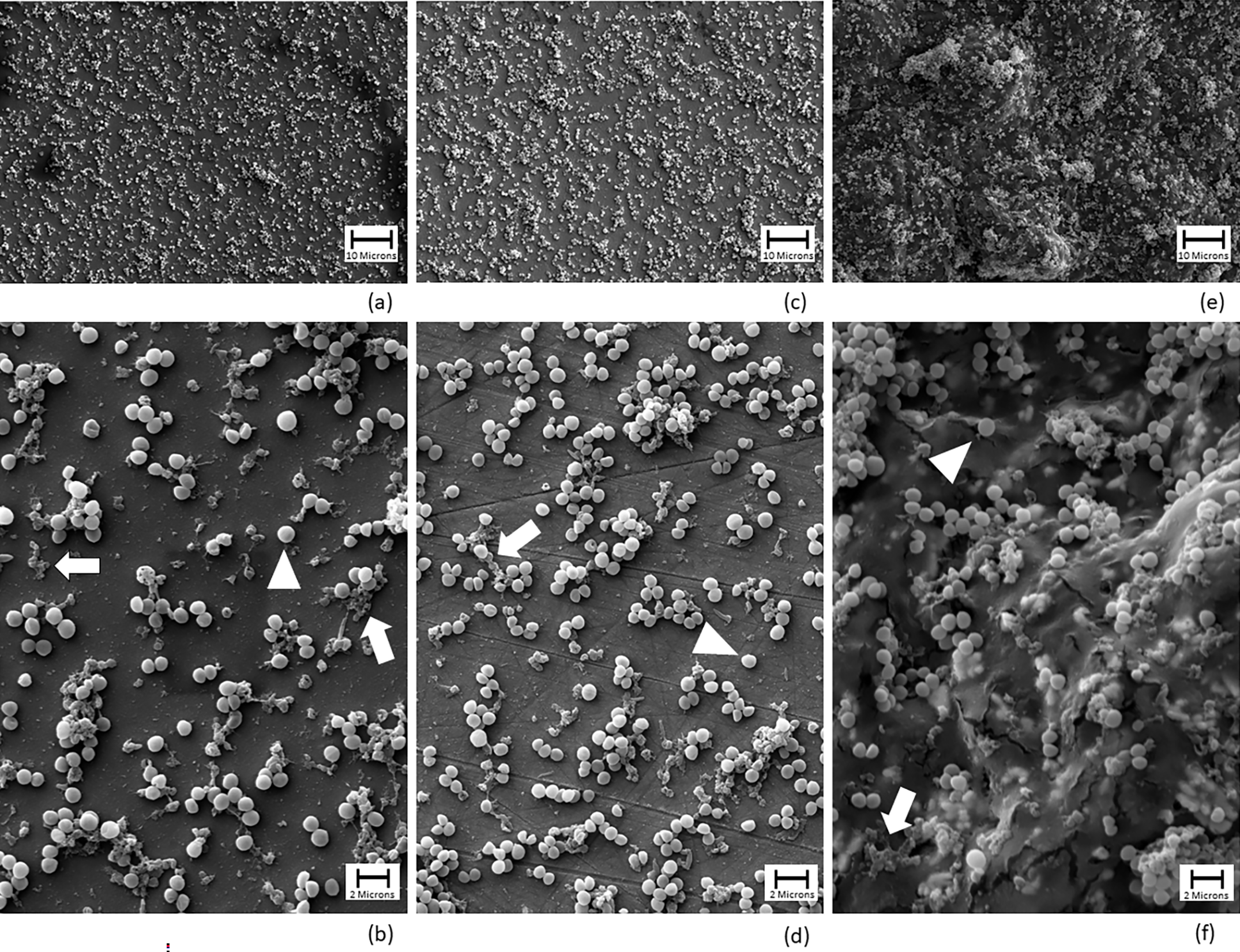
Antiseptic solutions are often used in total joint arthroplasty to help prevent or treat the challenging complication of periprosthetic joint infection. In a study now

New findings in musculoskeletal basic science are presented in the recent JBJS Guest Editorial What’s New in Musculoskeletal Basic Science. Here, we highlight the 5

Research is the foundation of countless achievements in science and medicine—and the shared calling of many in the orthopaedic community around the globe. In this
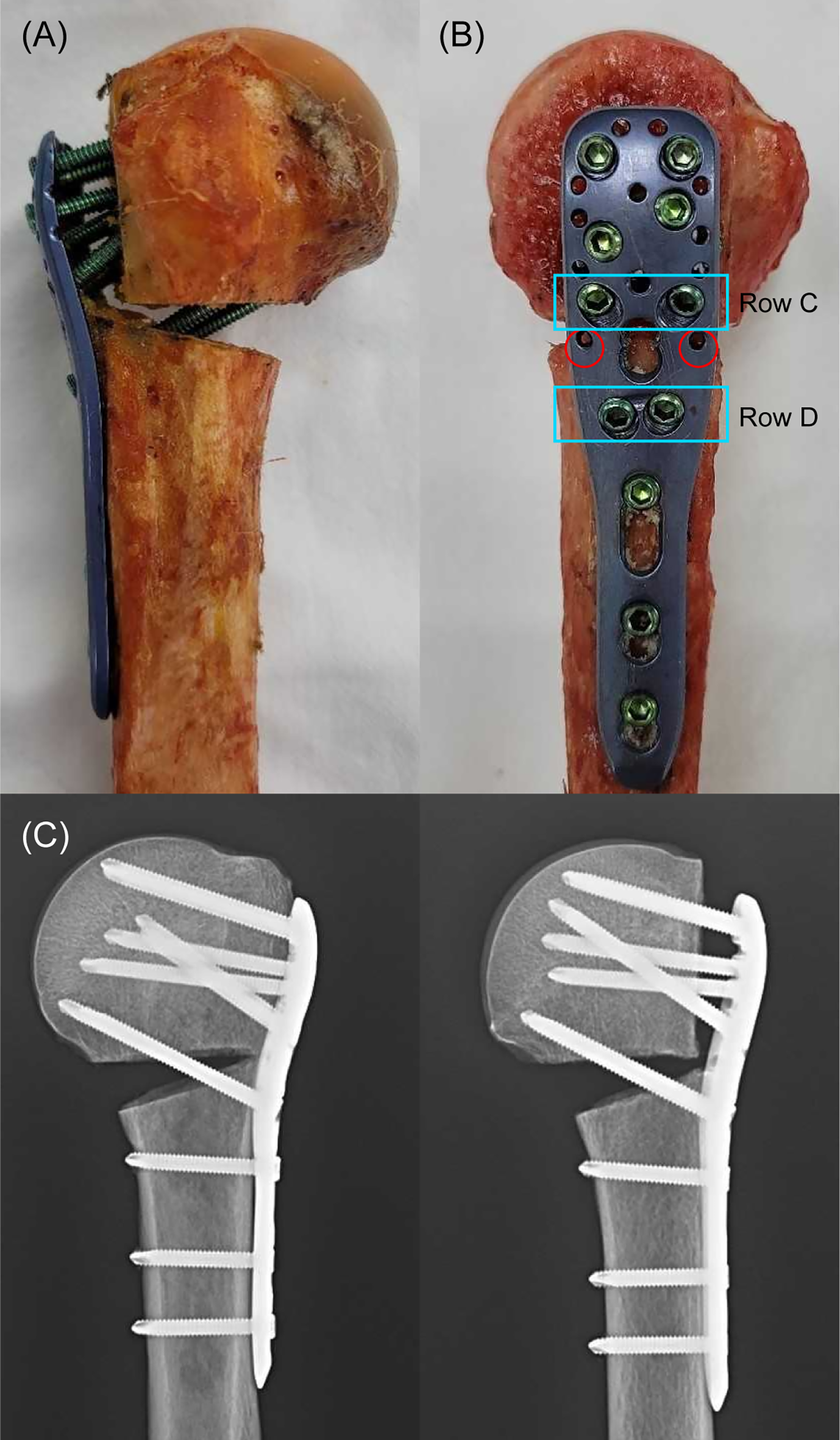
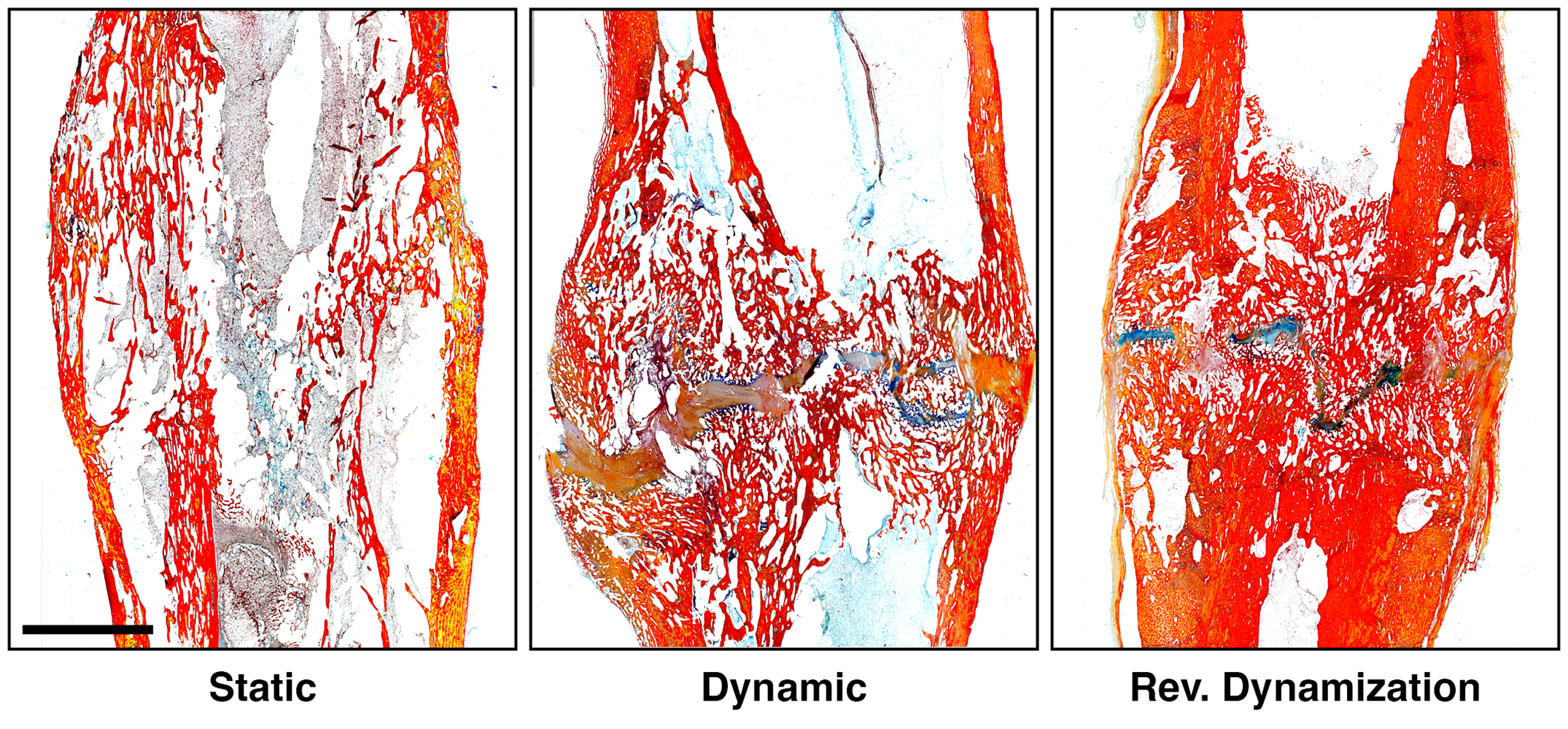
In this post, JBJS Deputy Editor for Social Media Dr. Matt Schmitz reflects on 2 recent studies of fracture fixation and the continued quest to
New findings in the field are presented in the recent JBJS Guest Editorial What’s New in Limb Lengthening and Deformity Correction. Here, we highlight the

Recent findings related to bone loss and bone healing, among other important topics, are presented in the new JBJS Guest Editorial What’s New in Musculoskeletal
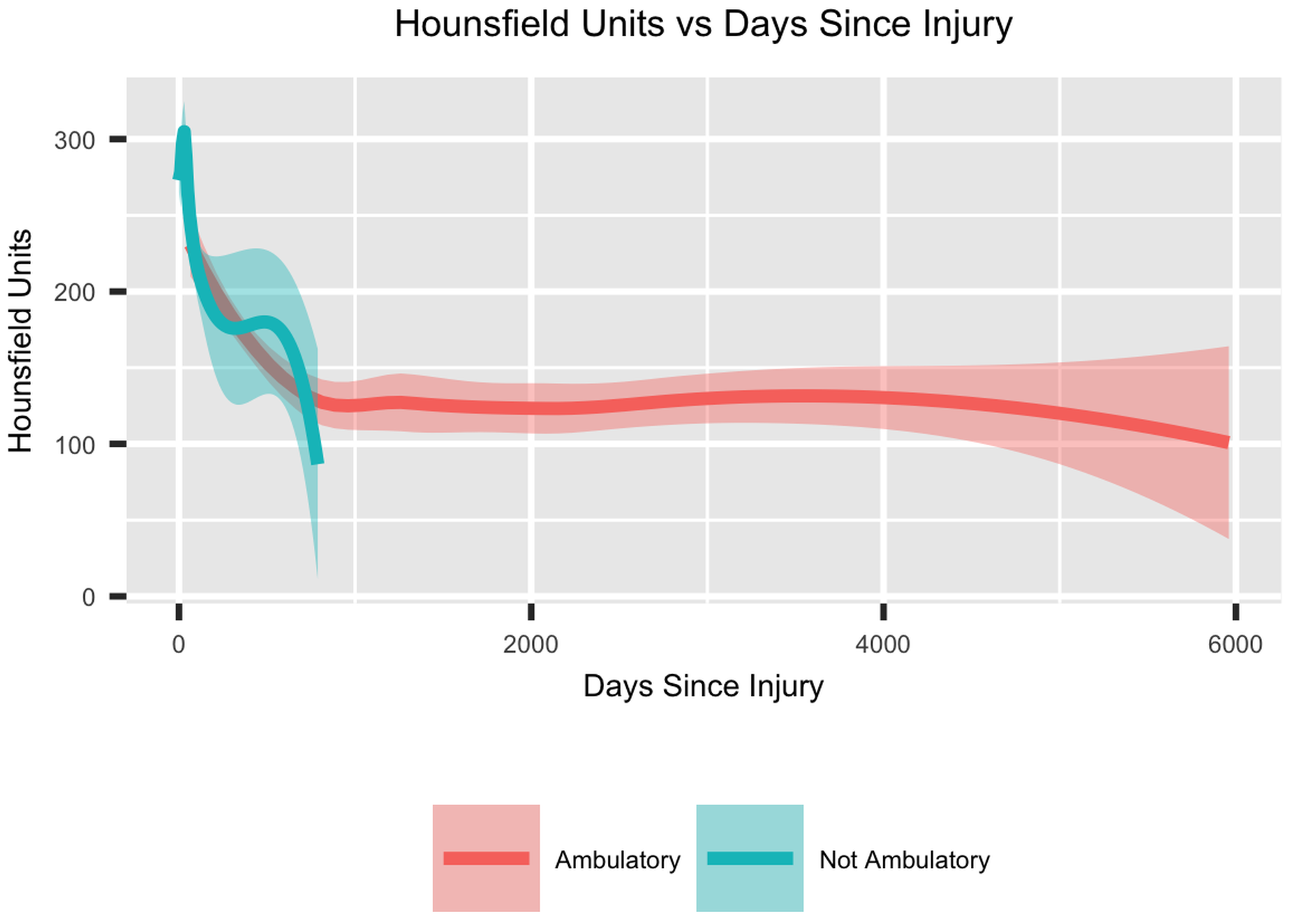
Patients who sustain major trauma are at risk of bone mineral density (BMD) loss, note Hoyt et al. in a new study in JBJS. After
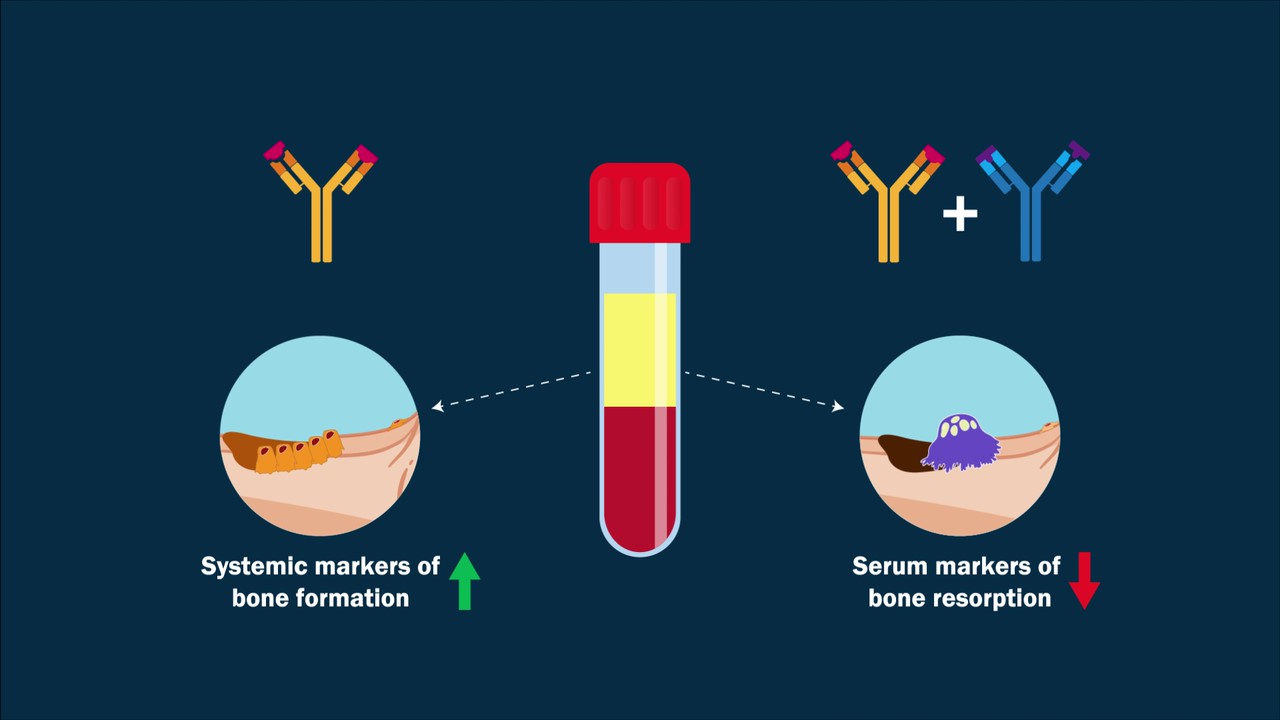
A video abstract is available with the new study by Florio et al. in JBJS: Dual Inhibition of the Wnt Inhibitors DKK1 and Sclerostin Promotes
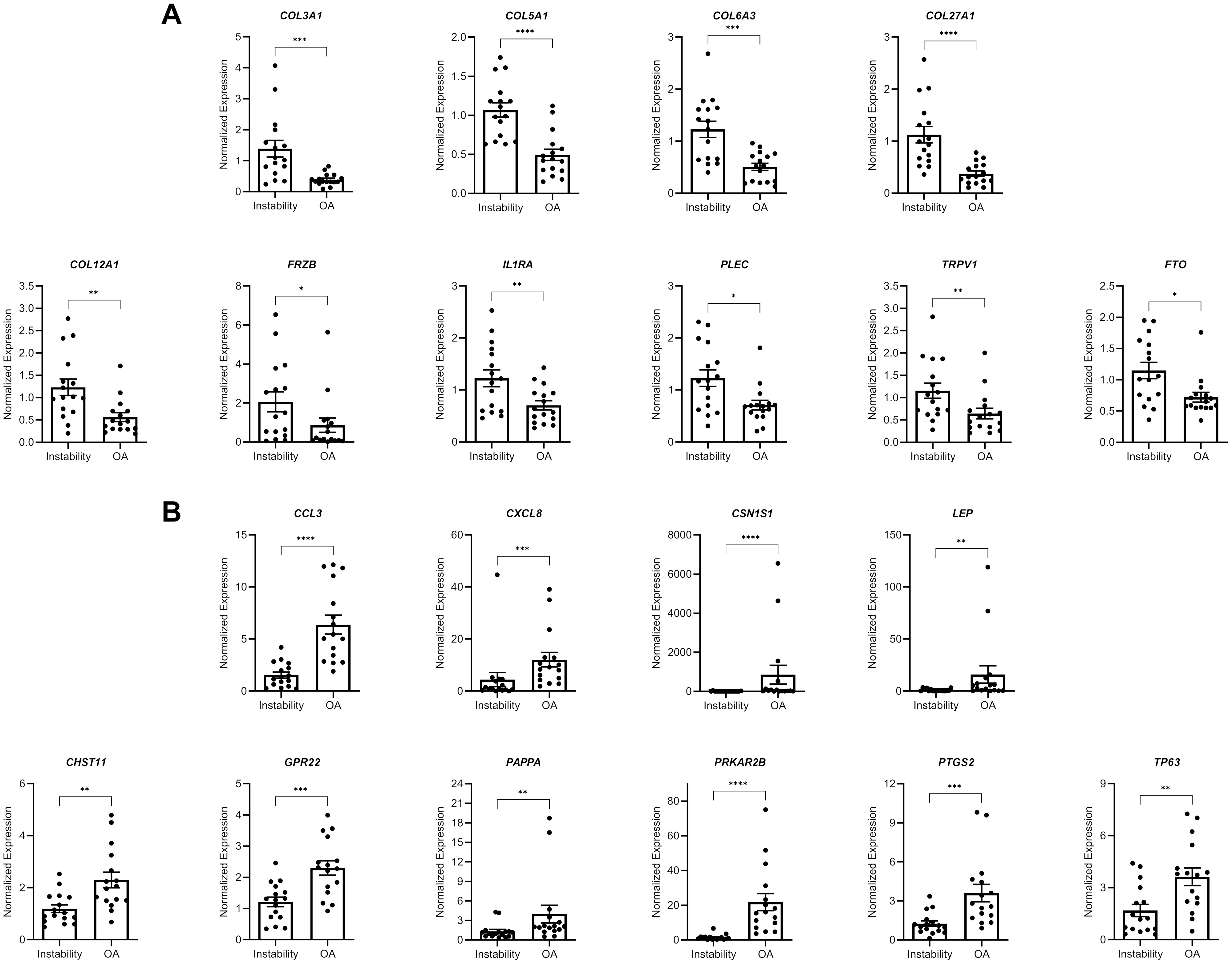
A new JBJS study presents novel data on gene expression in glenoid cartilage following shoulder instability. JBJS Deputy Editor for Social Media Dr. Matt Schmitz