OrthoBuzz is pleased to highlight the 2023 winners of the JBJS Open Access Awards. The annual awards were created in 2021 to acknowledge and celebrate the impactful
Category: Need to Know

In November 2023, leaders of the editorial boards of 6 orthopaedic journals, leaders of funding agencies, and officials from the National Institutes of Health met

JBJS is pleased to welcome Dr. Mohit (Mo) Bhandari as our new Editor-in-Chief. Dr. Bhandari succeeds Dr. Marc Swiontkowski, who has served as Editor-in-Chief since

The JBJS Clinical Summaries collection continues to expand. The summaries are a useful tool for musculoskeletal clinicians, offering synopses of recent findings and current treatment

The JBJS community extends its sincere thanks to Dr. Marc Swiontkowski for his steadfast and dedicated leadership as Editor-in-Chief over the past decade. As announced

“The first step in any effort to increase diversity is documenting the present state,” write Drs. Kanu Okike and Marc Swiontkowski in a new JBJS

In a recent article in JBJS, Escalera et al. provide an informative overview of the American Association of Latino Orthopaedic Surgeons (AALOS). Since its founding

OrthoBuzz marks the 1-year anniversary of JBJS OrthoCorps, an initiative that aims to preserve the voices and memories of the global orthopaedic community. In March

Interested in being published on the JBJS OrthoBuzz blog? Residents and trainees are invited to submit a post to Resident Roundup. Share your experience as
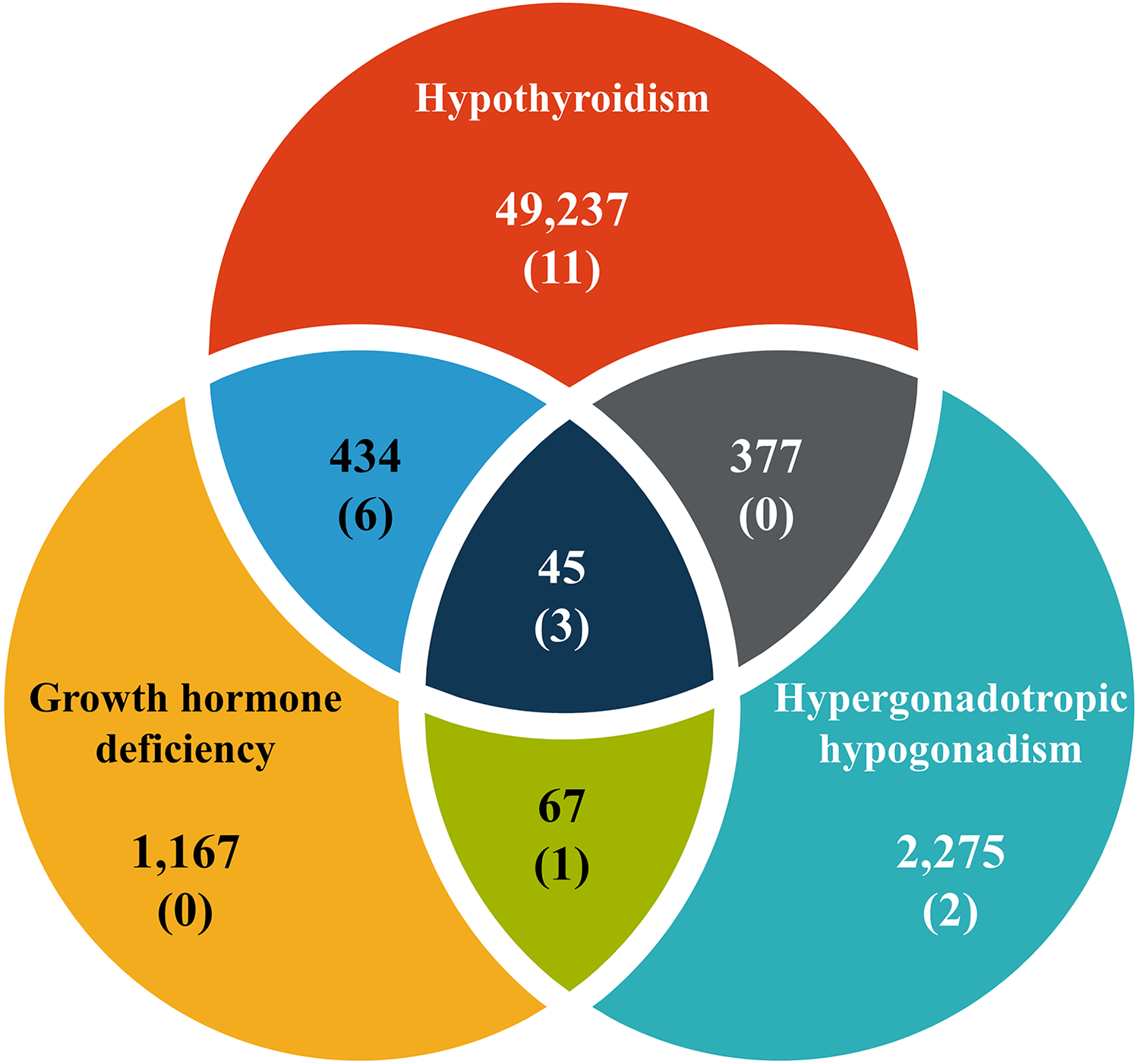
Endocrinopathy has been identified as a risk factor for slipped capital femoral epiphysis (SCFE). In young patients with endocrinopathy, what is the incidence of SCFE?

