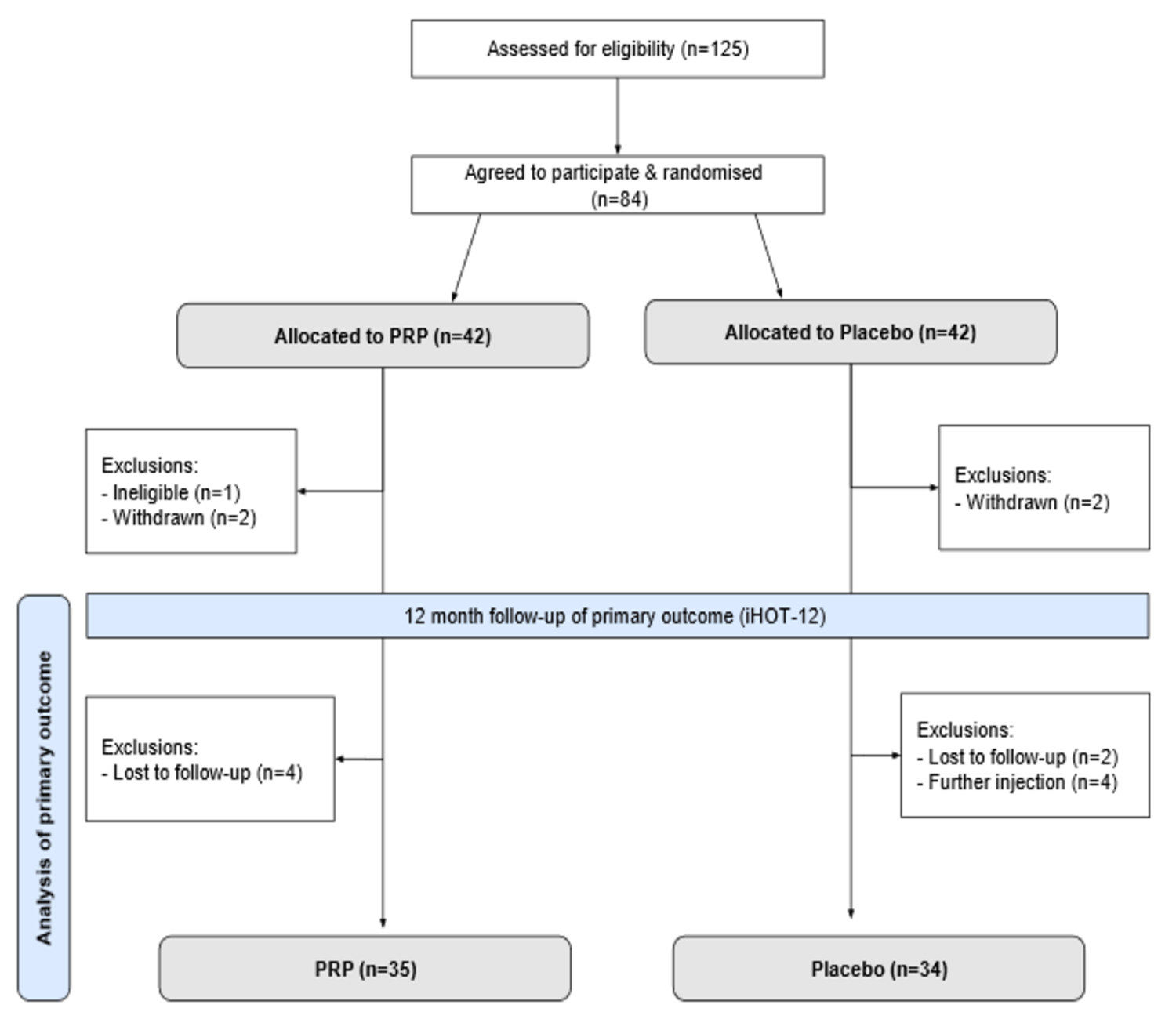
Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, highlights 2 new Level-I studies in total joint arthroplasty. Total knee arthroplasty (TKA) and total hip
Category: Hip
Key findings in sports medicine are highlighted in the new JBJS Guest Editorial What’s New in Sports Medicine. Here, we feature the 5 most impactful
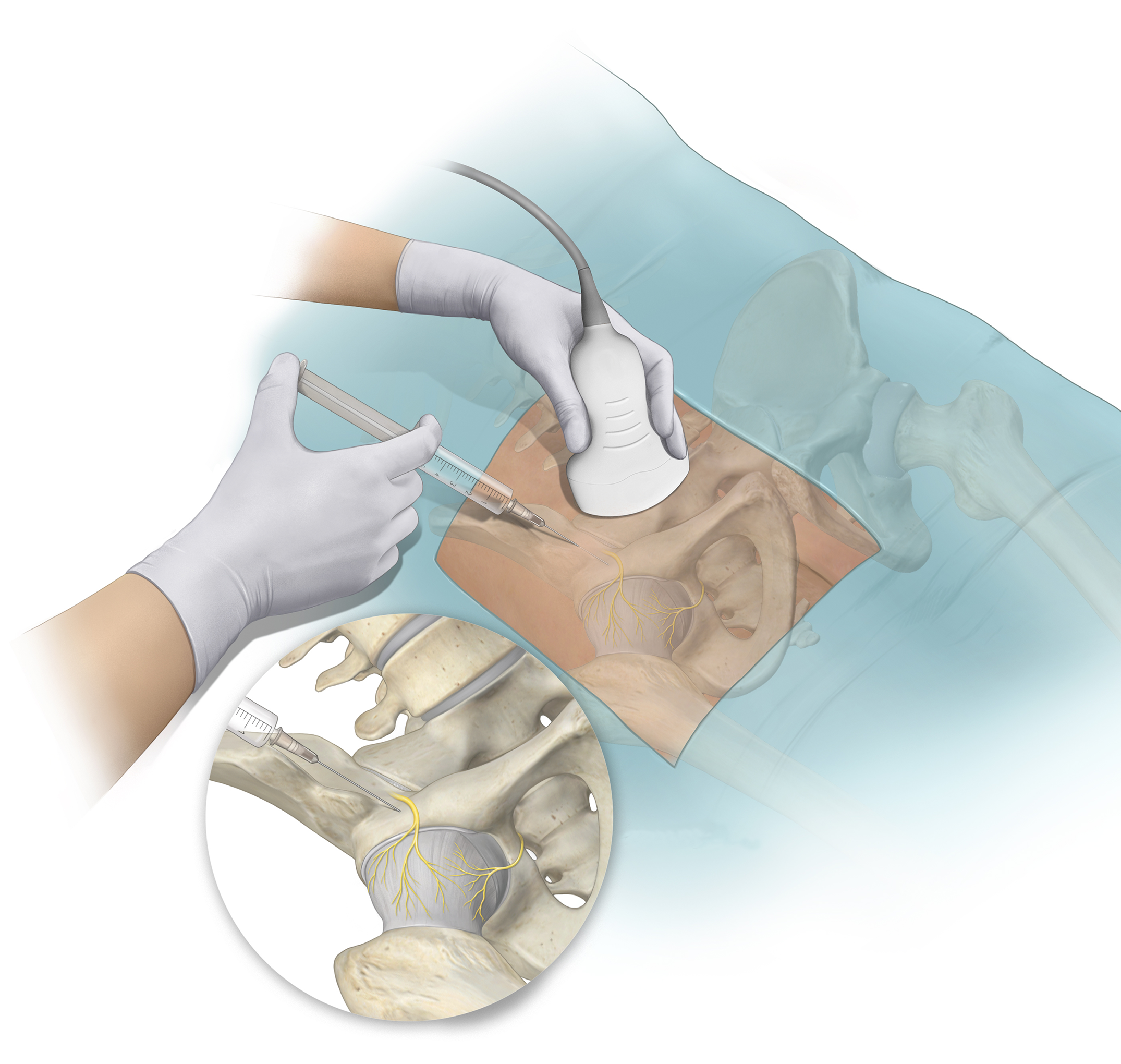
Dr. Matt Schmitz discusses 2 new RCTs investigating regional blocks in total knee and hip arthroplasty. The use of regional blocks in orthopaedic surgery has
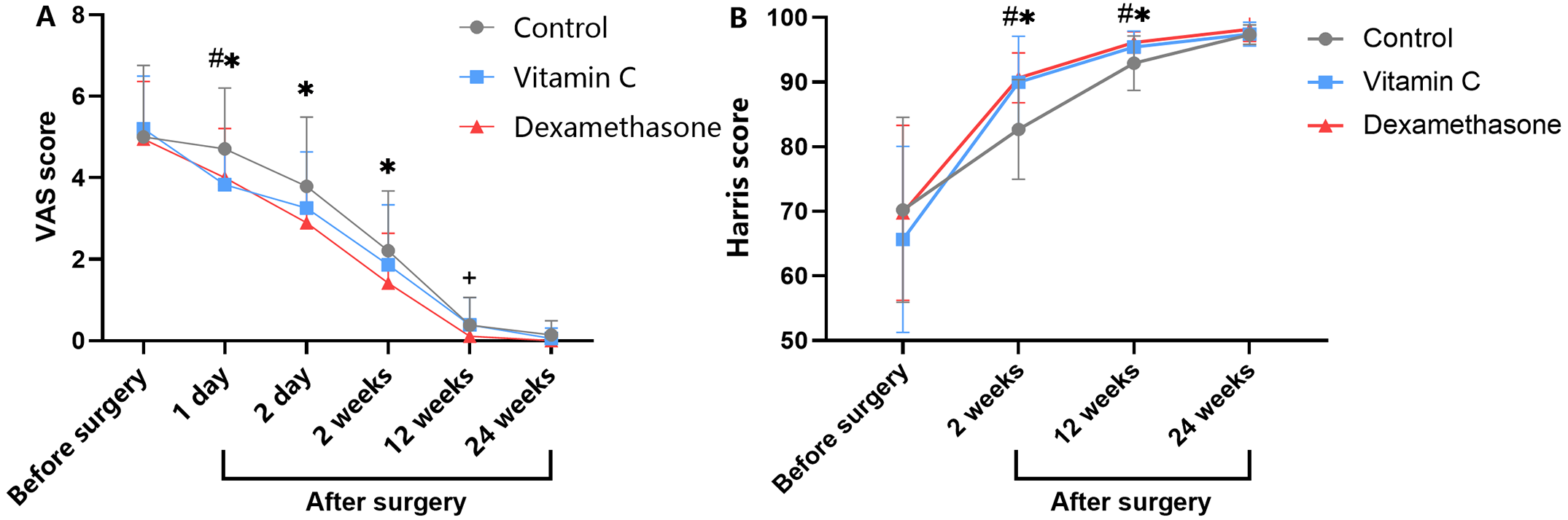
Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, offers this post on 2 level-I studies in the current issue of JBJS. In recent posts,
Notable findings in pediatric orthopaedics are presented in the JBJS Guest Editorial What’s New in Pediatric Orthopaedics. Here, we summarize the 5 most impactful studies,
In this post, Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, spotlights 3 total joint arthroplasty studies featured in the February 19, 2025 issue
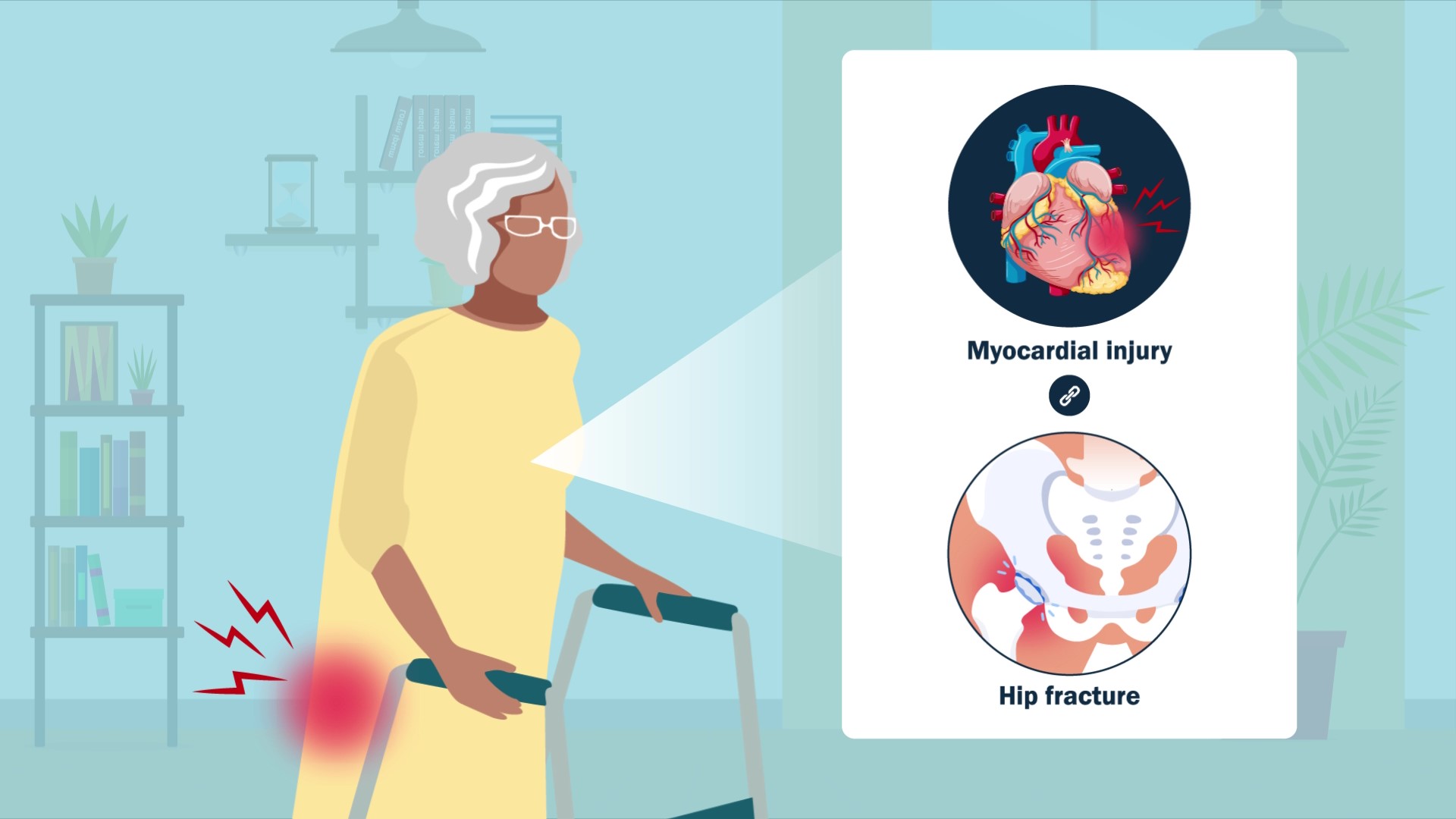
HIP ATTACK data show elevated troponin in 1 in 5 hip fracture patients, reports a new study in JBJS. For hip fracture patients with

A new special digital collection brings together the most-read JBJS Hip and Knee articles from the past 2 years. Including recent papers on hip and
Key findings in hip surgery, including those related to fracture management and infection prevention, are presented in the new JBJS Guest Editorial What’s New in
Early in my orthopaedic residency training, I remember an attending surgeon quizzing me on the mortality rate for elderly patients who sustained a hip fracture.