Key findings related to the prevention and treatment of periprosthetic joint infection (PJI), among other important topics, are presented in the new JBJS Guest Editorial
Category: Knee
Topics of interest in orthopaedic trauma, including fracture fixation, pain management, and more, are highlighted in the new JBJS Guest Editorial What’s New in Orthopaedic
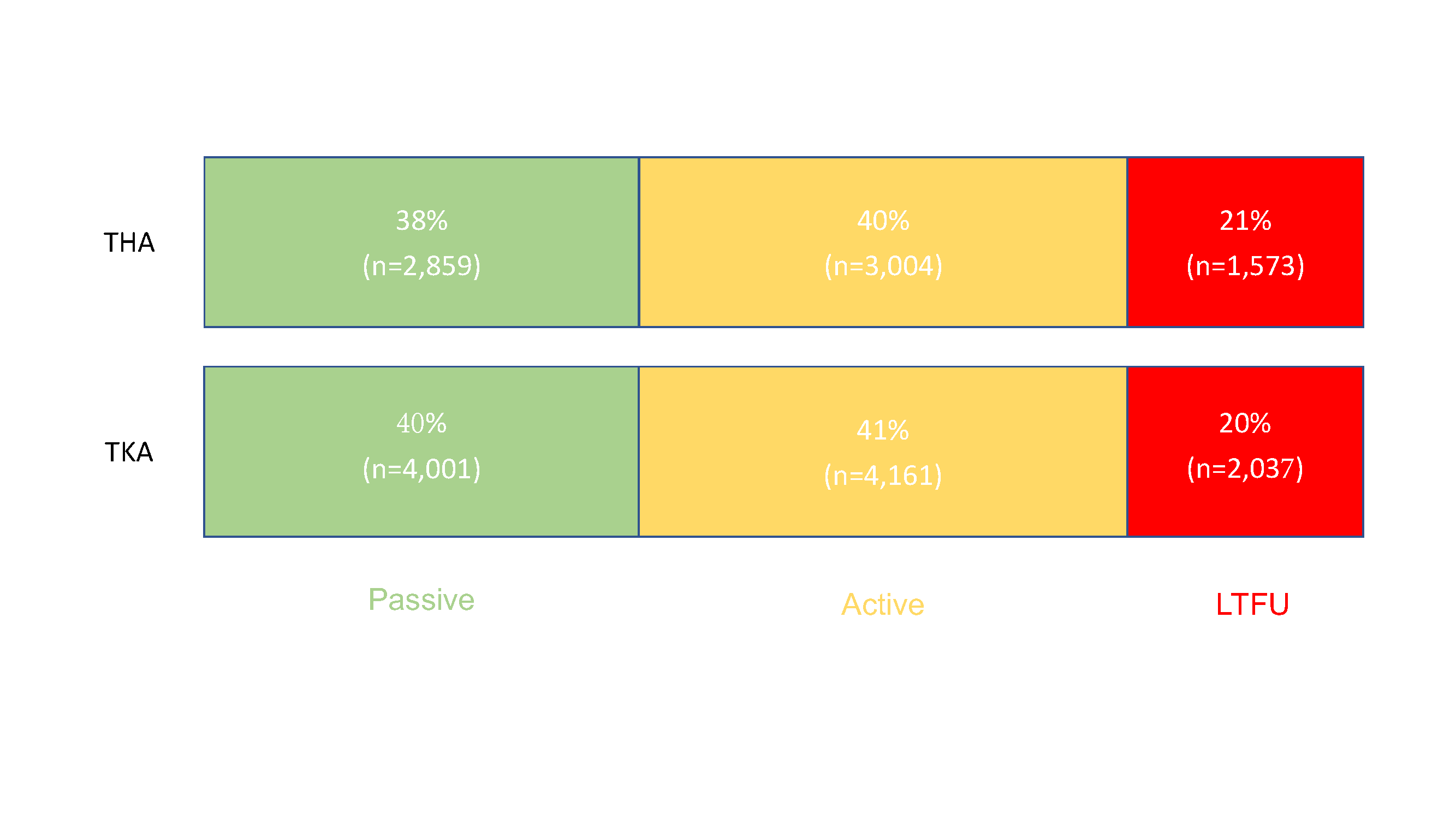
Editor-in-Chief Dr. Marc Swiontkowski reflects on a new study that examines the capture of patient-reported outcome measures (PROMs) after total joint arthroplasty. He offers a

Key findings in sports medicine on topics such as rotator cuff repair, anterior cruciate ligament (ACL) reconstruction, and more are presented in the new JBJS
JBJS Editor-in-Chief Dr. Marc Swiontkowski shares his thoughts on a new study showing the potential of preoperative bladder scanning to predict postoperative urinary retention in
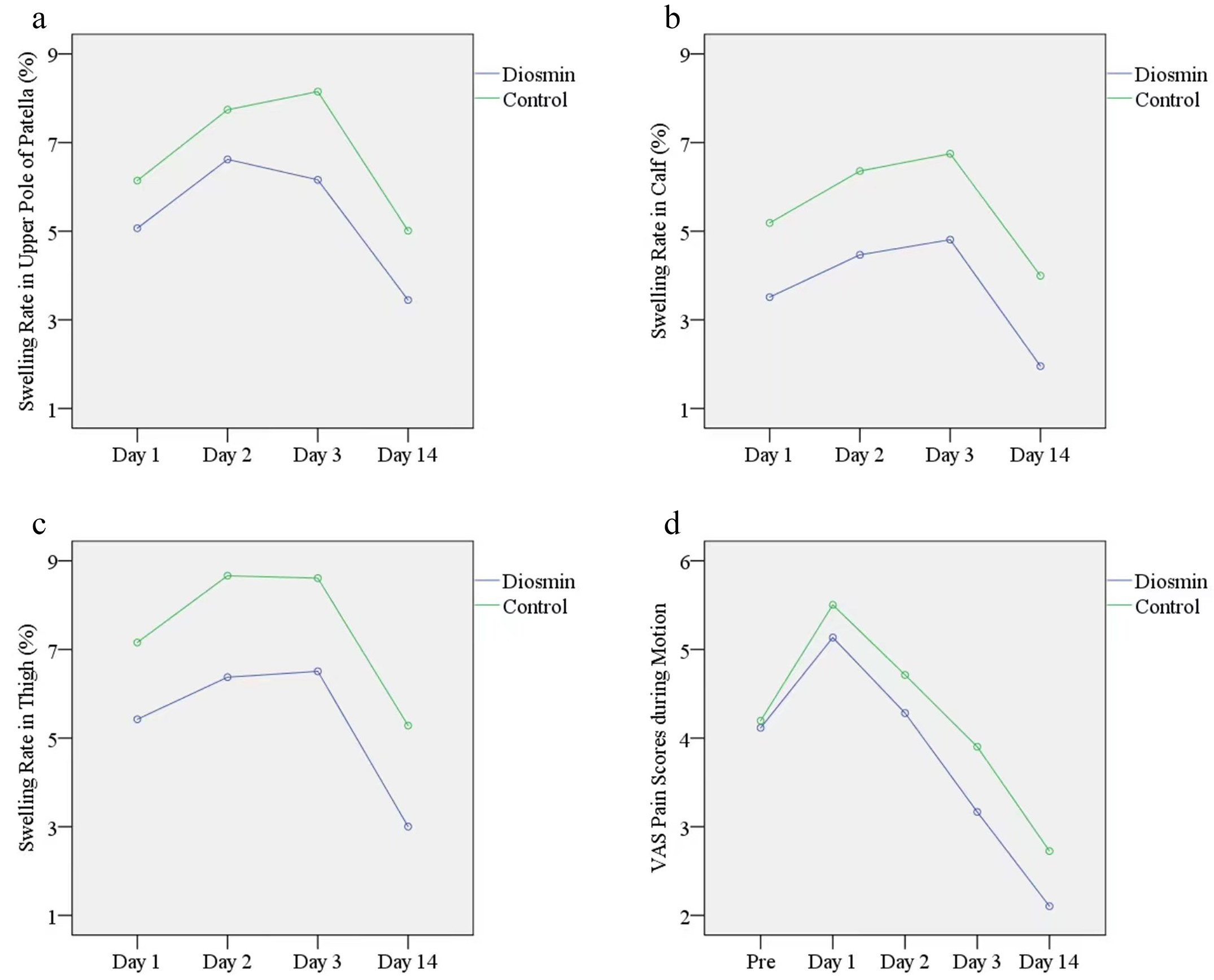
Editor-in-Chief Dr. Marc Swiontkowski offers this post on a new Level I study in JBJS evaluating the efficacy of diosmin in reducing swelling after total

A new study in JBJS investigates the relationship between outcomes following total knee arthroplasty (TKA) and social determinants of health. Two of the authors, Tahsin
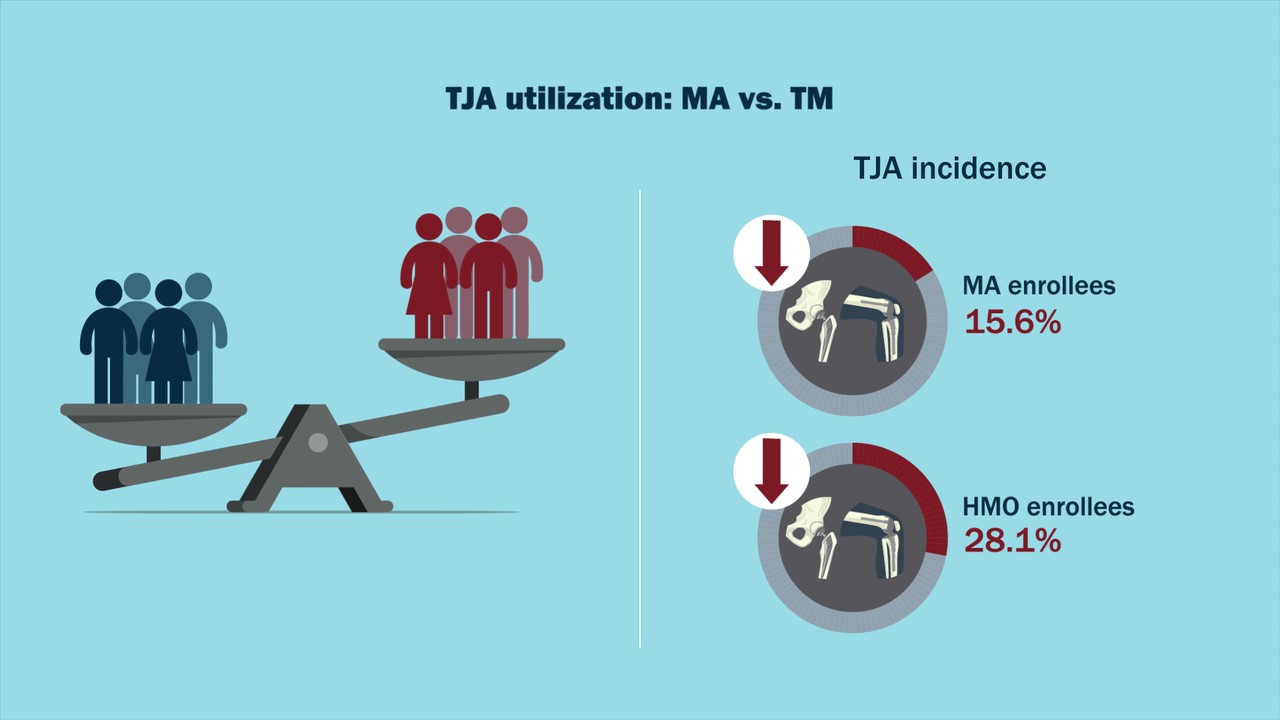
In a new study in JBJS, Anderson et al. provide enlightening data on total joint arthroplasty (TJA) utilization in the setting of Medicare Advantage (MA)
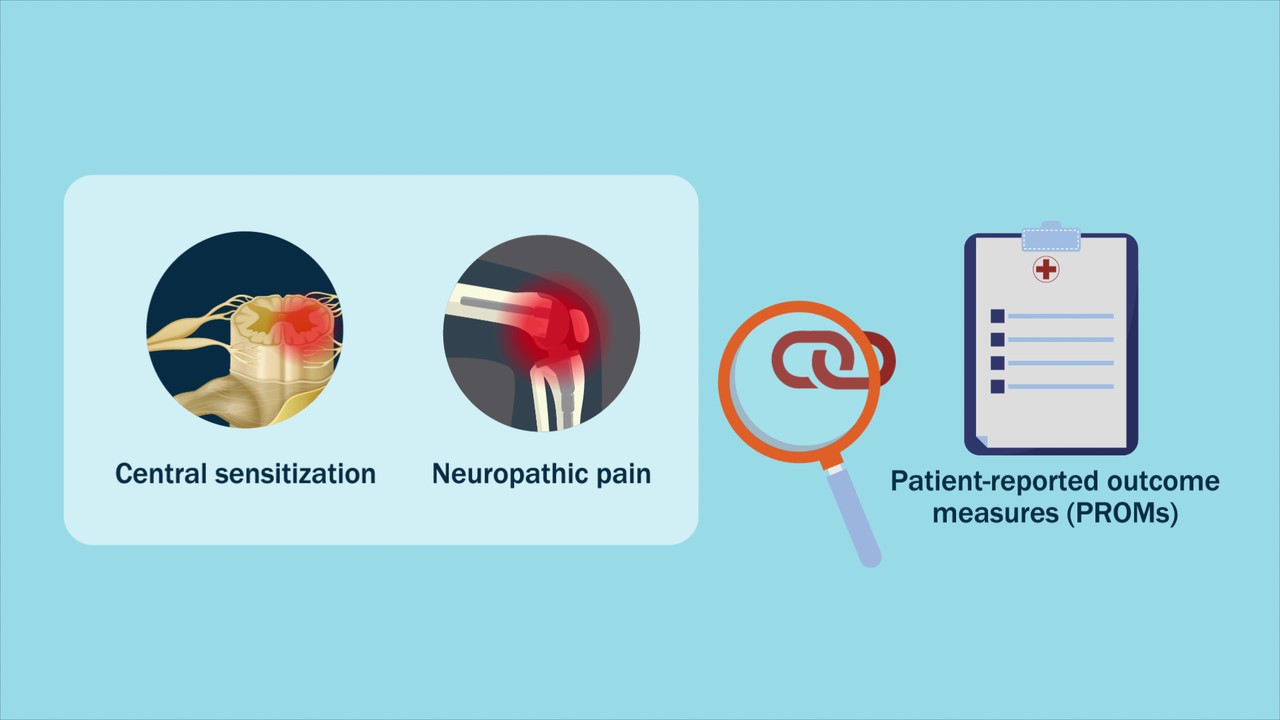
A video abstract is available with the new study by Kim et al. in JBJS: Central Sensitization and Neuropathic Pain Cumulatively Affect Patients Reporting Inferior
Findings from high-level and award-winning studies on topics such as unicompartmental and total knee arthroplasty (TKA), among others, are featured in the new JBJS Guest