The recent JBJS Guest Editorial “What’s New in Osteoporosis and Fragility Fractures” provides an update on this important area of orthopaedic research. The authors review
Tag: teriparatide
This post comes from Fred Nelson, MD, an orthopaedic surgeon in the Department of Orthopedics at Henry Ford Hospital and a clinical associate professor at
In the past several years, the orthopaedic community has become highly engaged in improving the follow-up management of patients presenting with fragility fractures. We have
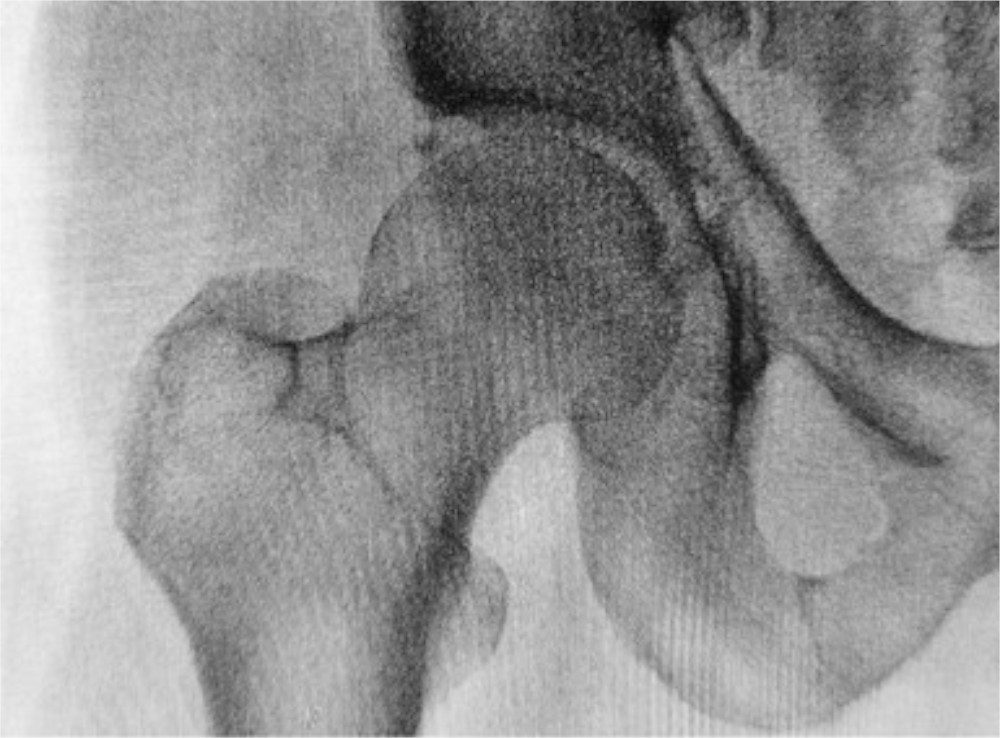
Physicians worldwide frequently prescribe bisphosphonates such as alendronate (Fosamax) and ibandronate (Boniva) to treat osteoporosis and prevent fragility fractures. Unfortunately, long-term bisphosphonate use has been