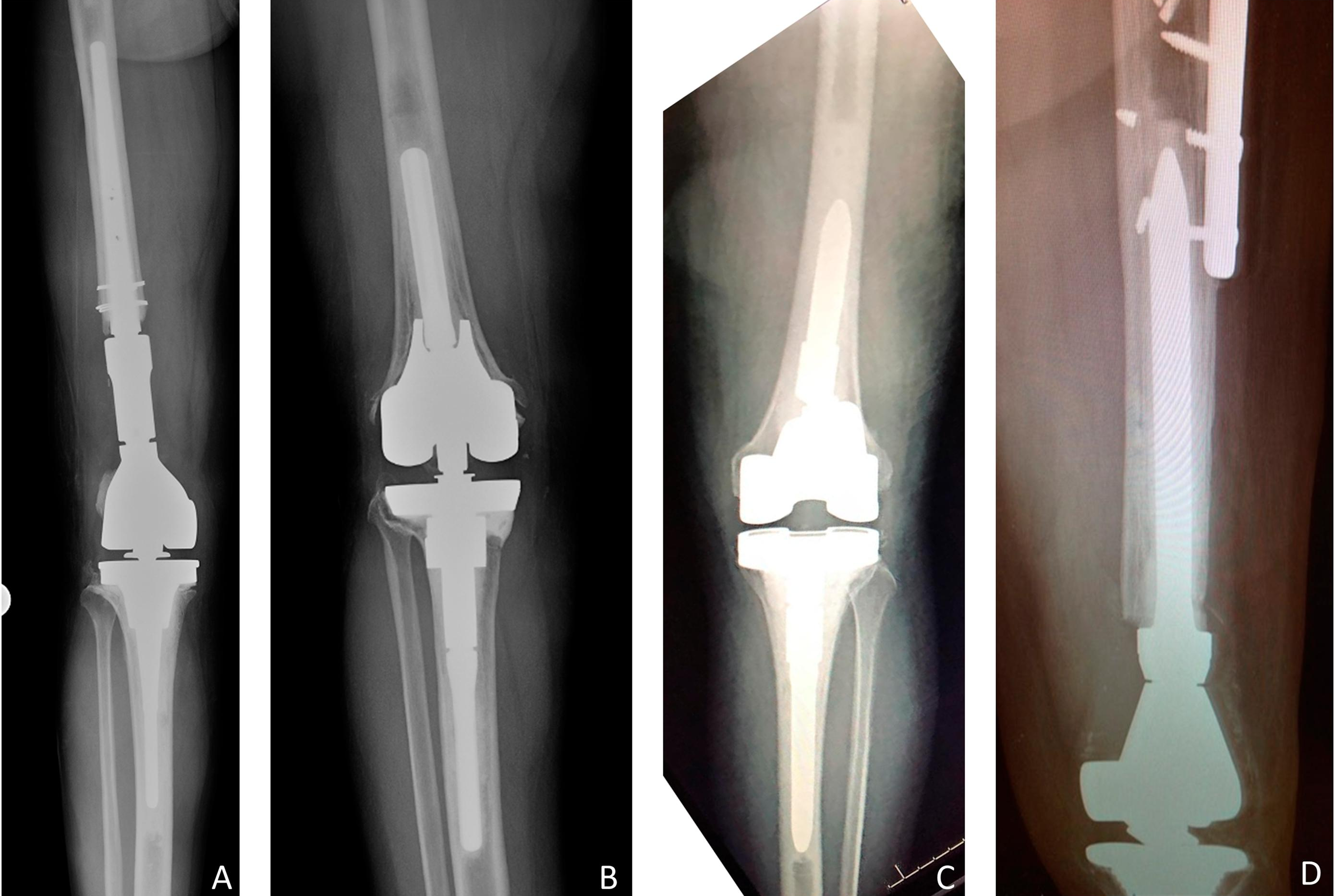
Recent studies on periprosthetic joint infection (PJI) and other topics are presented in the JBJS Guest Editorial “What’s New in Musculoskeletal Infection.” Here, we spotlight
Tag: vancomycin
Every month, JBJS publishes a review of the most pertinent and impactful studies from the orthopaedic literature during the previous year in 14 subspecialty areas. This month, co-author
Every month, JBJS reviews the most pertinent and impactful studies published in the orthopaedic literature during the previous year in 13 subspecialties. Click here for a collection
Every month, JBJS publishes a review of the most pertinent and impactful studies published in the orthopaedic literature during the previous year in 13 subspecialties. Click
The word “infection” contains 9 letters, but it’s a four-letter word for orthopaedic surgeons. Postoperative infections are complications that we all deal with, but we
Prompted by relatively high infection rates associated with surgical treatment of pediatric spinal conditions such as scoliosis and spinal-deformity surgery in immunocompromised adults, spine surgeons
Every month, JBJS publishes a review of the most pertinent and impactful studies published in the orthopaedic literature during the previous year in one of 13 subspecialties. Click
When it comes to preventing infections associated with orthopaedic procedures, the question of which antibiotic to use is only one of several concerns. How and
When >10% of patients undergoing procedures to correct a spinal deformity develop one or more surgical-site infections, investigations into how to mitigate such infections seem
Despite advances in sterile techniques and evidence-based use of perioperative antibiotics, periprosthetic joint infections still occur in 1% of primary and 3% to 7% of