All orthopaedic surgeons must carefully decide on which prophylactic antibiotic to prescribe when a patient or a young patient’s parent reports an allergy to penicillin. The specifics regarding the cross-reactivity of penicillin and first-generation cephalosporins are not fully understood, but the actual rate may be as low as 1 in 40,000 cases. Nonetheless, concerns regarding cross-reactivity generally lead to the use of a second-line prophylactic drug, such as clindamycin. Such decisions may not represent good antibiotic stewardship in these times when efforts to address antimicrobial resistance are dependent on the use of first-line prophylactic drugs whenever possible.
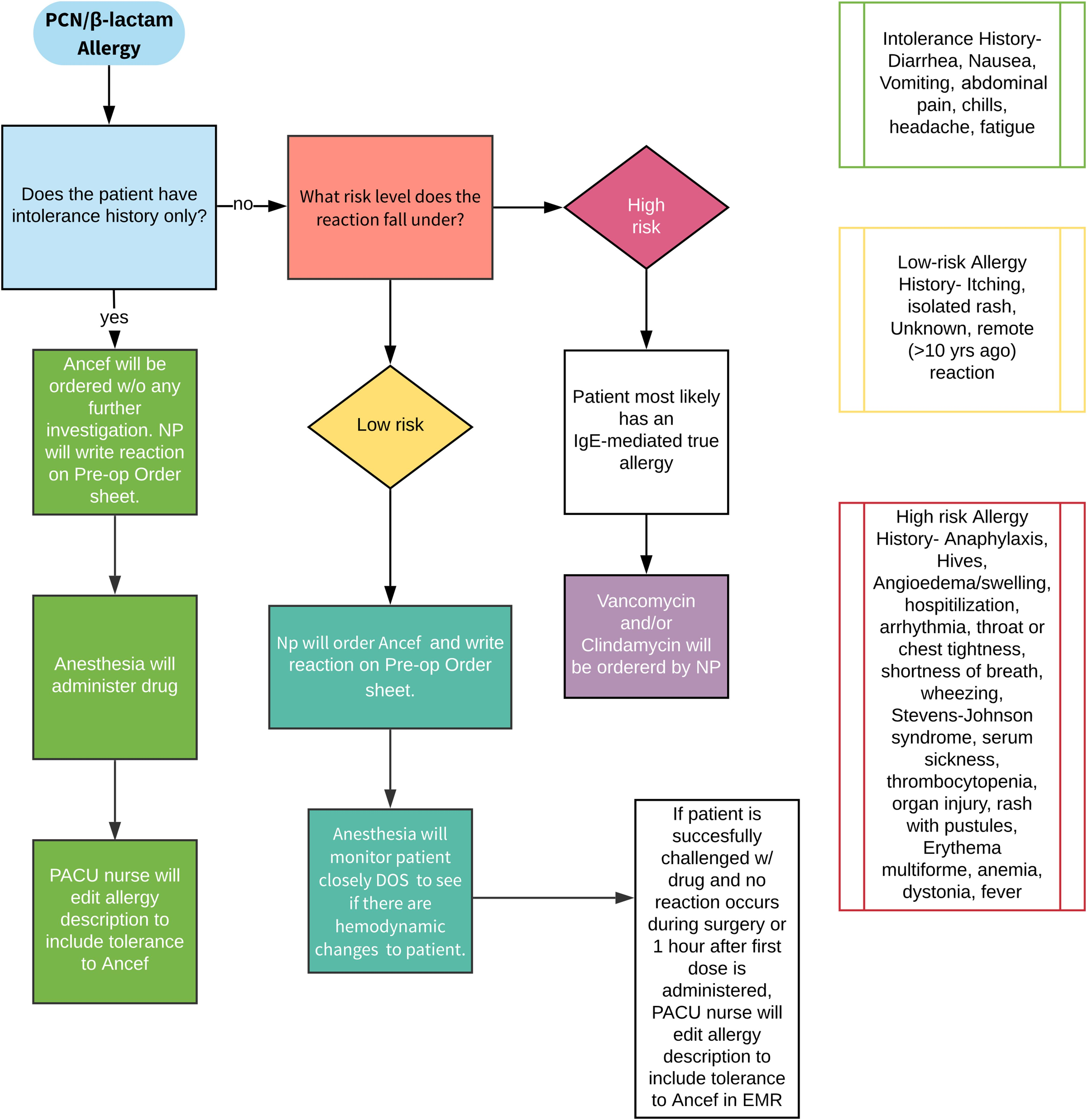
In the current issue of JBJS, Goh et al. report on a novel, questionnaire-based, penicillin allergy screening program developed to increase the use of cephalosporin prophylaxis in total joint arthroplasty (TJA) without the need for additional allergy testing. The protocol was developed by a multidisciplinary team including surgeons, medical consultants, and pharmacists.
Preadmission, participants received an educational handout explaining the low incidence of a true immunoglobin-E (IgE)-mediated allergic reaction among patients identified as having a penicillin allergy. Next, patients were given a questionnaire adapted from the American Medical Association Penicillin Allergy Toolkit, asking them to report whether they had a history of penicillin allergy. Those who reported an allergy were then asked about the details of the reaction.
Patients were then stratified into low-risk and high-risk groups; the low-risk group received cefazolin and the high-risk group received non-cefazolin antibiotics. A propensity score-matched control group of patients who underwent surgery before the implementation of the protocol was also included for comparison purposes.
- Of 2,078 patients in the protocol group, 357 patients (17.2%) reported a penicillin allergy on the questionnaire compared with 310 (14.9%) in the control group who had a penicillin allergy recorded in their electronic health record (p = 0.052).
- In the protocol group, 66.9% were categorized as low-risk and given cefazolin despite a self-reported penicillin allergy; only 3 (1.3%) experienced a mild cutaneous reaction after receiving cefazolin.
- The number of patients who received non-cephalosporin antibiotics was significantly lower in the protocol group than in the control group (5.7% vs. 15.2%; p < 0.01). Multiple logistic regression analysis demonstrated that patients in the protocol group were 3 times more likely to receive cefazolin.
- The rate of adverse reactions to any antibiotic did not differ before and after the screening protocol was implemented (0.7% vs. 0.8%; p = 0.857). A total of 31 allergic reactions were noted across the control and protocol groups; all required only antihistamines for treatment. The rates of superficial wound infections, deep infections, and C. difficile infections did not differ between the control and protocol groups.
This study provides immensely valuable data on the use of a simple, algorithmic approach for the safe use of cephalosporin in TJA patients with a reported penicillin allergy. I agree with the opinion shared by Thomas Parker Vail, MD in a related commentary article that “accepting the limitations [of the study] and applying this algorithm within the stated inclusion criteria would still move the needle of prophylactic antibiotic use in a very positive direction.”
Click here for the full report.
A downloadable JBJS Infographic summarizing the study can be found here.
Marc Swiontkowski, MD
JBJS Editor-in-Chief