A randomized clinical trial remains at the top of the Level of Evidence pyramid precisely because this experimental design is the optimum way to limit bias and assure that the compared populations are as close to equivalent as possible. While this experimental design is difficult to implement, we have seen it increasingly used in orthopaedic trials, with slight but steady increases in the percentage of manuscripts published annually in the literature. When we first began to employ this design in the 1980s, we experienced a very high incidence of underpowered trials being published. We have improved as a surgical research community with the understanding of statistical fragility—the identification of the often small number of subjects for whom an alternate outcome could potentially flip the results of the trial. We now understand the benefit of trials with a large sample, generally with multicenter involvement, to help assure the generalizability of the effectiveness of findings. Noninferiority trial designs are now part of our common lexicon.
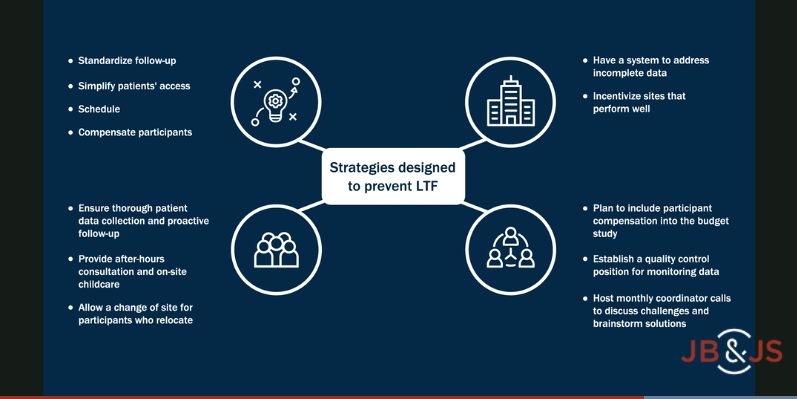
Apart from these trial design considerations, patient loss to follow-up (LTF) is the next critical issue. LTF can easily dampen the impact of a randomized trial, if not completely render the conclusions unreliable. In the current issue of JBJS, Firth et al. critically examine the LTF issue, analyzing a cohort of 618 young, active patients enrolled in the STABILITY 1 study, a randomized controlled trial comparing anterior cruciate ligament (ACL) reconstruction with or without a lateral extra-articular tenodesis. The study was conducted at 7 Canadian and 2 European centers.
Patients were followed for 2 years postoperatively, with clinic visits and 9 patient-reported outcome measures completed at 3, 6, 12, and 24 months. The rate of LTF was 8.3% (51 patients: 17 with early LTF and 34 with late LTF). A total of 77 patients (12.5%) missed the final clinical assessment.
The authors identified that current or previous smoking, part-time employment, and a body mass index of ≥25 kg/m2 were significant predictors of LTF. Most importantly, in my opinion, was the center effect on the study findings. Access the full report here.
In today’s society, young adults are often mobile, changing locations based on educational, employment, and family demands. In my personal experience in conducting prospective trials, nothing ensures follow-up more that the attending surgeon being involved with the patient in trial recruitment and consistently reiterating to the patient and their family that participation in the trial will help future patients with the same condition or injury. Working to establish a personal connection with the patient and family with this shared goal is the best prevention for LTF. This effort, combined with requesting contact information for a relative or friend in the event the patient cannot be reached, can help with reconnection. Collectively, we all need to work to limit LTF, as it can negate the positive impact of trial results for patients, families, and researchers trying to improve the care of tomorrow’s orthopaedic patients.
Marc Swiontkowski, MD
JBJS Editor-in-Chief
A JBJS Video Summary of this study is available here.