Vitamin D supplementation, fracture risk, and osteoporosis drug therapies are among several topics explored in recent studies featured in the new JBJS Guest Editorial: What’s New in Osteoporosis and Fragility Fractures. Here, we highlight the 5 most compelling studies, as selected by co-author Joseph M. Lane, MD.
Vitamin D Supplementation
In an ancillary study of the Vitamin D and Omega-3 Trial (VITAL) trial, investigators evaluated the influence of supplemental vitamin D3 vs. placebo on incident fractures1. The trial population included U.S. men ≥50 years of age and women ≥55 years of age. Of note, participants were not recruited because of vitamin D deficiency, low bone mass, or osteoporosis. Supplemental vitamin D3 was not demonstrated to have a significant effect on total fractures, nonvertebral fractures, or hip fractures. Write the authors of the JBJS Guest Editorial, “This study did not substantiate a benefit of vitamin D in healthy, middle-aged individuals. It did not refute the benefit in individuals with marked vitamin D deficiency (<15 ng/mL). Thus, the recommendation of achieving a 25(OH) vitamin D level of approximately 30 to 35 ng/mL may still be reasonable, especially in patients with documented osteoporosis and a history of fragility fractures.”
Fracture Risk
In a recent study in the U.K., investigators found that proton pump inhibitor (PPI) use was associated with decreased bone mineral density (BMD) in lumbar spine and total hip regions analyzed2. The gut microbiome did not appear to contribute to this effect. The data suggest that metabolites from PPI ingestion may influence bone density by way of plasma metabolites involved in the sex hormone pathway. The study called on data from >5,000 individuals in the TwinsUK cohort.
Osteoporosis Drug Therapy
Findings from a study in Denmark help to elucidate the challenge of discontinuing denosumab given the “rebound phenomenon.” Investigators assessed changes in the receptor activator of nuclear factor kappa-B ligand (RANKL) and tartrate-resistant acid phosphatase 5b (TRAcP 5b) after discontinuation of long-term denosumab3. A total of 61 patients were randomized to treatment with zoledronate at 6, 9, or 12 months after the last denosumab injection or when bone turnover was high. The data suggest that RANKL stimulation of osteoclasts may lead to increased bone resorption and ensuing risk of vertebral fracture.
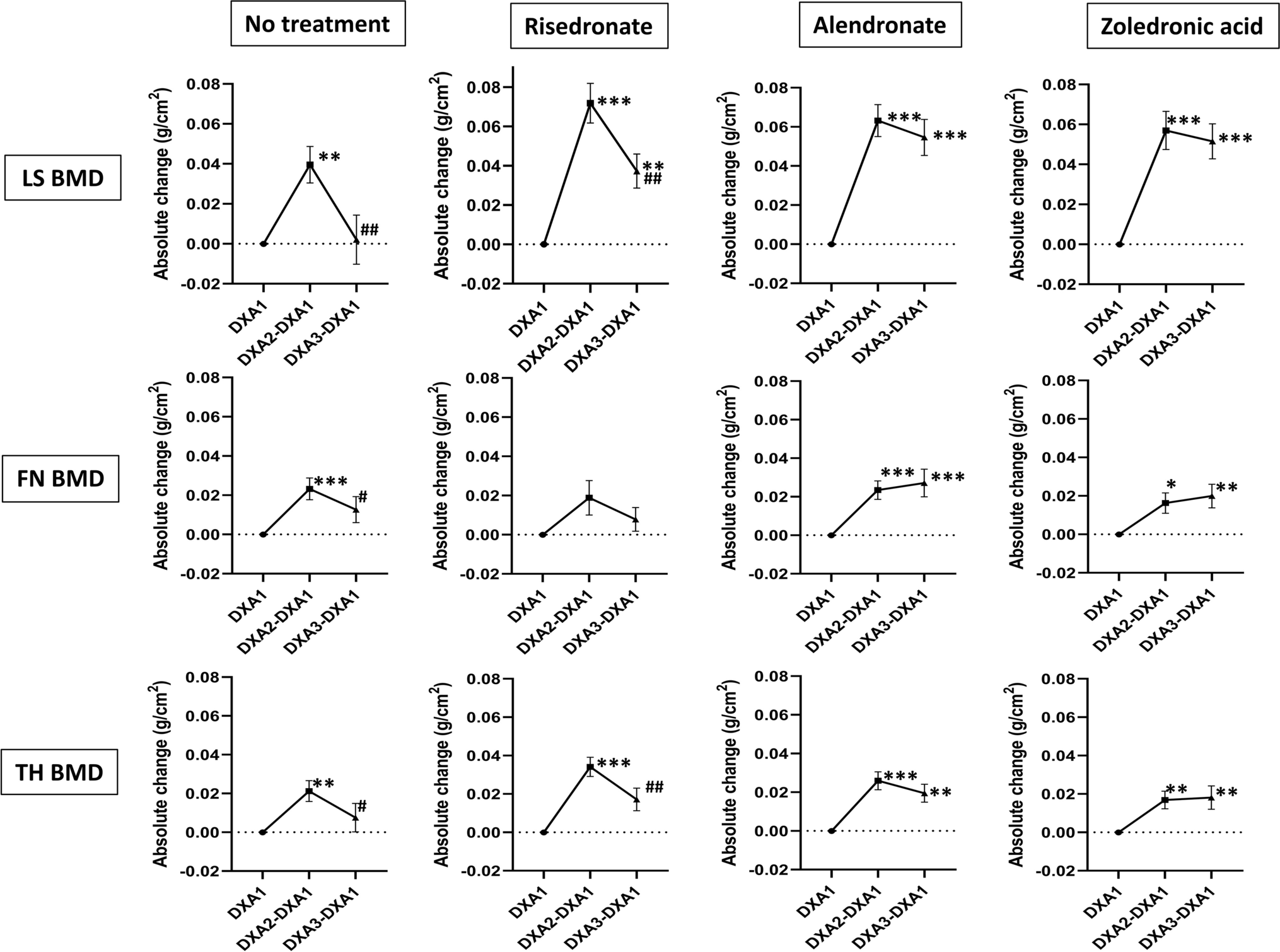
In another study, investigators performed a retrospective analysis of 121 patients who discontinued denosumab followed by no treatment, risedronate, alendronate, or zoledronic acid4. In assessing changes in lumbar spine bone density, the researchers found differences between the no-treatment and alendronate group and the no-treatment and zoledronic acid group. Regarding BMD in the total hip, only the comparison between the no-treatment and zoledronic acid group yielded a significant difference. Five of seven vertebral fractures occurred in the group with no treatment. The authors concluded that BMD loss during the rebound phase after discontinuation of denosumab is mitigated by treatment with alendronate or zoledronic acid for 6 months.
Lastly, a team of researchers in Taiwan conducted a population-based study comparing various anti-osteoporosis medications in terms of post-fracture mortality5. A total of 46,729 individuals (average age of 74.45 years, 80% female) were enrolled. The investigators concluded that the use of osteoporotic medication, particularly long-acting, may lower post-fracture mortality.
What’s New in Osteoporosis and Fragility Fractures is freely available at JBJS.org.
What’s New by Subspecialty
Each month, JBJS publishes a review of the most pertinent studies from the orthopaedic literature in a select subspecialty. To read the reports, visit the What’s New by Subspecialty collection at JBJS.org.
Recent OrthoBuzz posts include: What’s New in Limb Lengthening and Deformity Correction, What’s New in Musculoskeletal Infection, and What’s New in Orthopaedic Trauma.
Image from: Tutaworn T, Nieves JW, Wang Z, Levin JE, Yoo JE, Lane JM. Bone loss after denosumab discontinuation is prevented by alendronate and zoledronic acid but not risedronate: a retrospective study. Osteoporos Int. 2023 Mar;34(3):573-84. © The Author(s) 2023. https://creativecommons.org/licenses/by-nc/4.0/
References
- LeBoff MS, Chou SH, Ratliff KA, Cook NR, Khurana B, Kim E, Cawthon PM, Bauer DC, Black D, Gallagher JC, Lee IM, Buring JE, Manson JE. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022 Jul 28;387(4):299-309.
- Zhang X, Adebayo AS, Wang D, Raza Y, Tomlinson M, Dooley H, Bowyer RCE, Small KS, Steves CJ, Spector TD, Duncan EL, Visconti A, Falchi M. PPI-induced changes in plasma metabolite levels influence total hip bone mineral density in a UK cohort. J Bone Miner Res. 2023 Feb;38(2):326-34.
- Sølling AS, Harsløf T, Jørgensen NR, Langdahl B. Changes in RANKL and TRAcP 5b after discontinuation of denosumab suggest RANKL mediated formation of osteoclasts results in the increased bone resorption. Osteoporos Int. 2023 Mar;34(3):599-605.
- Tutaworn T, Nieves JW, Wang Z, Levin JE, Yoo JE, Lane JM. Bone loss after denosumab discontinuation is prevented by alendronate and zoledronic acid but not risedronate: a retrospective study. Osteoporos Int. 2023 Mar;34(3):573-84.
- Wu CH, Li CC, Hsu YH, Liang FW, Chang YF, Hwang JS. Comparisons between different anti-osteoporosis medications on postfracture mortality: a population-based study. J Clin Endocrinol Metab. 2023 Mar 10;108(4):827-33.