Recent findings in orthopaedic oncology and musculoskeletal tumor management are highlighted in the new JBJS Guest Editorial What’s New in Musculoskeletal Tumor Surgery. Here, we highlight the 5 most compelling studies, as selected by co-author Michelle Ghert, MD, FRCSC.
Bone Tumor Outcomes
Minimally important differences (MIDs) for the Musculoskeletal Tumor Society score and the Toronto Extremity Salvage Score were established for patients with lower-extremity bone tumors undergoing endoprosthetic reconstruction1. The study, a secondary analysis of the PARITY trial, is available at JBJS.org. The Musculoskeletal Tumor Society score had an anchor-based MID of 12 points and a distribution-based MID of 16 to 17 points. The Toronto Extremity Salvage Score had an anchor-based MID of 11 points and a distribution-based MID of 14 points. The investigators recommend use of the anchor-based MIDs, “as they are grounded in changes in functional status that are relevant and meaningful to patients.”
Soft-Tissue Sarcoma Therapies
The efficacy and safety of using the immunotherapy agents durvalumab plus tremelimumab in patients with advanced or metastatic bone or soft-tissue sarcomas was investigated2. A total of 57 adult patients (≥18 years old) received treatment in this single-center, Phase-2 study. Progression-free survival at 12 weeks was observed in 49% of the patients. The results of this study demonstrate “modest anti-tumor activity with manageable side effects,” note Guest Editorial authors Dr. Gazendam and Dr. Ghert.
The wound complication rate was assessed among adult patients with nonmetastatic soft-tissue sarcoma who underwent a hypofractionated radiation therapy regimen for 3 weeks preoperatively in a single-center, Phase-2 trial3. Postoperatively, a major wound complication occurred in 37 of 120 patients. Good overall survival outcomes and acceptable toxicity levels were reported. The researchers concluded that the regimen was safe and could be more convenient for patients than conventional treatment.
Novel Approaches to Soft-Tissue Tumors
A Phase-3, double-blinded, randomized controlled trial evaluated the use of nirogacestat, a γ-secretase inhibitor, in adults with desmoid tumors4. A total of 142 patients received either a placebo (72 patients) or 150 mg of oral nirogacestat (70 patients) twice daily. Progression-free survival, the primary outcome, was significantly better in the intervention group (hazard ratio, 0.29; p < 0.001) compared with the control group. Additionally, the response rate was significantly higher in the intervention group.
A secondary analysis of the ENLIVEN trial was conducted to assess the efficacy of pexidartinib for pain relief5. In the ENLIVEN trial, patients with tenosynovial giant cell tumors not likely to improve with surgery were administered pexidartinib or a placebo for 24 weeks. Exploratory analyses conducted as part of the secondary analysis detected a modest improvement in pain relief associated with pexidartinib.
What’s New in Musculoskeletal Tumor Surgery is freely available at JBJS.org.
Related reading on OrthoBuzz: JBJS Supplement on the Secondary Analyses from the PARITY Trial
What’s New by Subspecialty
Each month, JBJS publishes a review of the most pertinent studies from the orthopaedic literature in a select subspecialty. To read the reports, visit the What’s New by Subspecialty collection at JBJS.org.
Recent OrthoBuzz posts include: What’s New in Musculoskeletal Basic Science, What’s New in Orthopaedic Rehabilitation, and What’s New in Shoulder and Elbow Surgery.
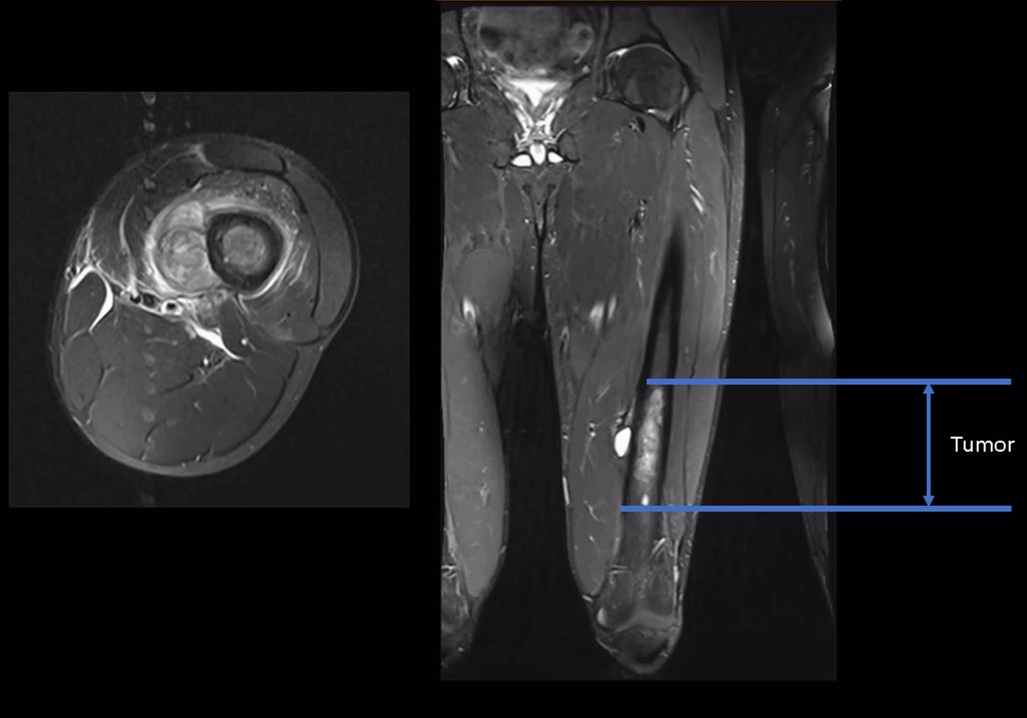
Image reproduced from: Gundavda MK, Lazarides AL, Burke ZDC, Tsoi K, Ferguson PC, Wunder JS. Reconstructive allograft preparation for long bone intercalary segments after tumor resections: Toronto Sarcoma protocol. JBJS Essential Surgical Techniques. 2023;13(2):e22.00011.
References
- Gazendam AM, Schneider P, Bhandari M, Busse JW, Ghert M; on behalf of the PARITY Investigators. Defining minimally important differences in functional outcomes in musculoskeletal oncology. J Bone Joint Surg Am. 2022 Sep 21;104(18):1659-66.
- Somaiah N, Conley AP, Parra ER, Lin H, Amini B, Solis Soto L, Salazar R, Barreto C, Chen H, Gite S, Haymaker C, Nassif EF, Bernatchez C, Mitra A, Livingston JA, Ravi V, Araujo DM, Benjamin R, Patel S, Zarzour MA, Sabir S, Lazar AJ, Wang WL, Daw NC, Zhou X, Roland CL, Cooper ZA, Rodriguez-Canales J, Futreal A, Soria JC, Wistuba II, Hwu P. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: a single-centre phase 2 trial. Lancet Oncol. 2022 Sep;23(9):1156-66.
- Guadagnolo BA, Bassett RL, Mitra D, Farooqi A, Hempel C, Dorber C, Willis T, Wang WL, Ratan R, Somaiah N, Benjamin RS, Torres KE, Hunt KK, Scally CP, Keung EZ, Satcher RL, Bird JE, Lin PP, Moon BS, Lewis VO, Roland CL, Bishop AJ. Hypofractionated, 3-week, preoperative radiotherapy for patients with soft tissue sarcomas (HYPORT-STS): a single-centre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2022 Dec;23(12):1547-57.
- Gounder M, Ratan R, Alcindor T, Schöffski P, van der Graaf WT, Wilky BA, Riedel RF, Lim A, Smith LM, Moody S, Attia S, Chawla S, D’Amato G, Federman N, Merriam P, Van Tine BA, Vincenzi B, Benson C, Bui NQ, Chugh R, Tinoco G, Charlson J, Dileo P, Hartner L, Lapeire L, Mazzeo F, Palmerini E, Reichardt P, Stacchiotti S, Bailey HH, Burgess MA, Cote GM, Davis LE, Deshpande H, Gelderblom H, Grignani G, Loggers E, Philip T, Pressey JG, Kummar S, Kasper B. Nirogacestat, a γ-secretase inhibitor for desmoid tumors. N Engl J Med. 2023 Mar 9;388(10):898-912.
- Healey JH, Tap WD, Gelhorn HL, Ye X, Speck RM, Palmerini E, Stacchiotti S, Desai J, Wagner AJ, Alcindor T, Ganjoo K, Martín-Broto J, Wang Q, Shuster D, Gelderblom H, van de Sande M. Pexidartinib provides modest pain relief in patients with tenosynovial giant cell tumor: results from ENLIVEN. Clin Orthop Relat Res. 2023 Jan 1;481(1):107-16.