JBJS Deputy Editor for Social Media Dr. Matt Schmitz offers his thoughts on a new study comparing the efficacy of opioid and non-opioid analgesia following closed reduction and percutaneous pinning (CRPP) of supracondylar humeral fractures in pediatric patients.
The opioid epidemic has been well documented in both the medical literature and public media. It is vital that surgeons safely shift away from knee-jerk prescriptions of potentially addictive medications for postoperative pain control, especially for common pediatric fracture diagnoses. Previous retrospective studies have called into question the need for opioids after closed reduction and percutaneous pinning (CRPP) of supracondylar humeral fractures, but there has been a dearth of high-quality prospective analyses.
In the current issue of JBJS, Belardo et al. report the findings of a Level II prospective, comparative cohort study in which they evaluated the efficacy of opioid vs. non-opioid analgesia after CRPP of supracondylar humeral fractures in pediatric patients. Their report is now available at JBJS.org:
Eligible for inclusion were children 3 to 12 years of age who were treated with CRPP for a closed, modified Gartland Type-II or III supracondylar humeral fracture. The median age at the time of the procedure was 6.2 years.
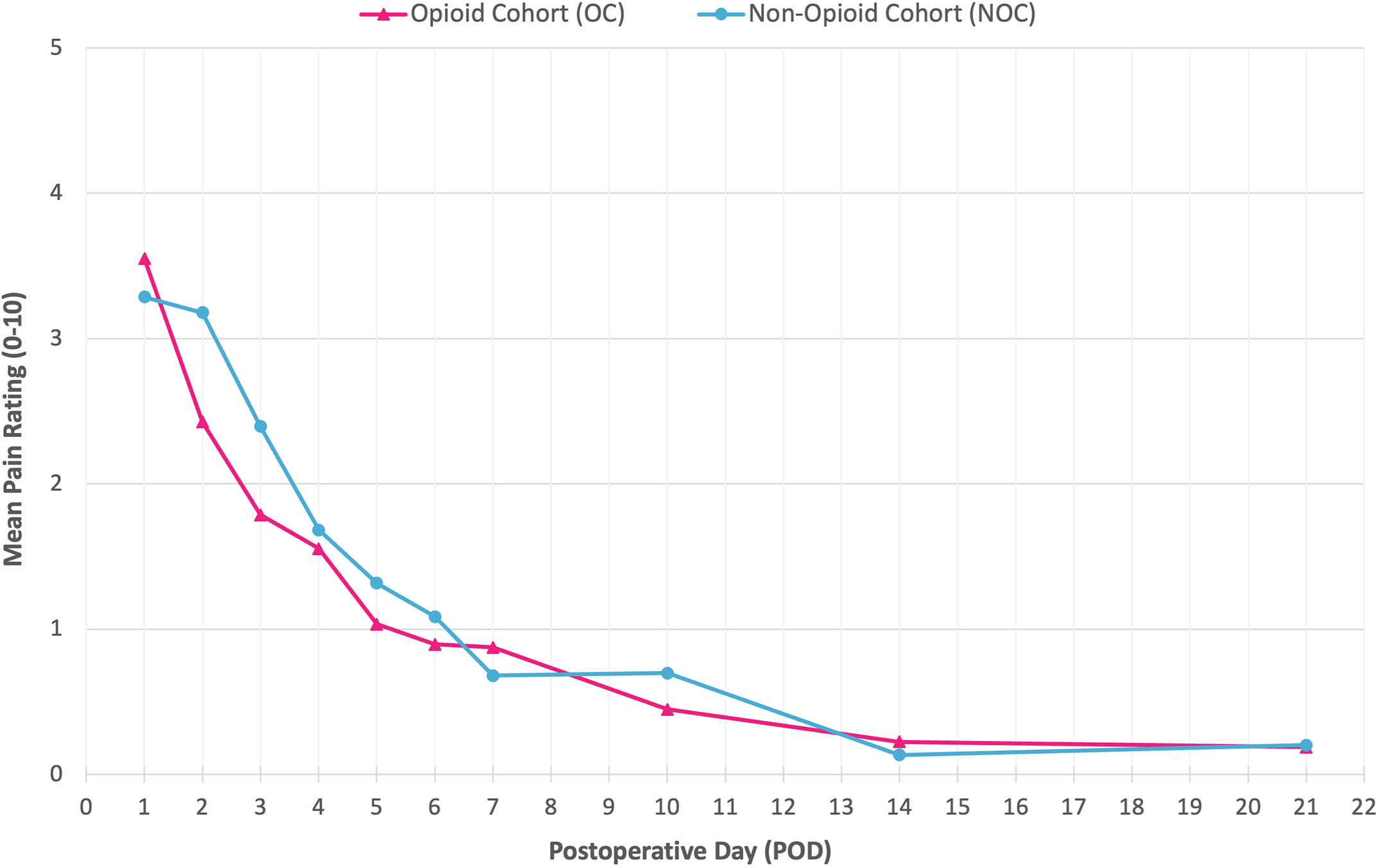
Four U.S. tertiary children’s hospitals participated. At 2 of the hospitals, the patients were prescribed oxycodone, acetaminophen, and ibuprofen at discharge (the opioid cohort), with standard instructions to utilize oxycodone only for breakthrough pain and with 7 doses of narcotic prescribed. Patients at the other 2 hospitals were prescribed acetaminophen and ibuprofen alone (non-opioid cohort). Over 16 months, 157 children were evaluated: 48.4% were in the non-opioid cohort and 51.6% were in the opioid cohort. Caregivers received text-message questionnaires on postoperative days 1 through 7 as well as on days 10, 14, and 21 to document the patient’s pain and medication doses.
Patient and Operative Data
- The 2 cohorts did not differ significantly in terms of age, sex, race, or body mass index. The non-opioid cohort included more Hispanic and Latino patients than the opioid cohort (33% compared with 11%; p < 0.001), but ethnicity was not correlated with mean pain scores or total medication use.
- The procedure time was statistically greater in the opioid vs. non-opioid cohort (34 vs. 27 minutes; p = 0.006), but the clinical importance of this can be debated.
- No significant correlations were found between postoperative pain and procedure time, number of pins used, pin configuration, or type of immobilization.
Pain Ratings and Analgesia Use
- The investigators found no significant differences in pain ratings between the non-opioid cohort and opioid cohort from postoperative days 1 to 21.
- The number of upper outlier pain scores (scores >95th percentile in the data set) did not differ between the 2 cohorts.
- In the opioid group, 28 (34.6%) of 81 children took no oxycodone, while 40 children (49.4%) took 1 to 3 total doses, with opioids rarely used after postoperative day 2.
Kudos to all involved for prospectively studying the need for opioid analgesia in pediatric supracondylar humeral fracture treatment. This study demonstrated that access to opioids did not impact the mean postoperative pain that the patients experienced, and opioid intake was very low in the group that had access based on prescribing patterns.
In a related Author Insights video, co-author Dr. Apurva Shah says that the finding that pain relief was not better for children who received opioid vs. non-opioid medicine has led to a change in treatment at his institution. Access the video here: Author Insights by Apurva S. Shah, MD, MBA.
In their report, the authors comment that “reducing the amount of opioids prescribed, via standardized order sets and family education, is critical to reduce opioid misuse.” I wholeheartedly agree. Data from this study indicate that non-opioid analgesia is effective in controlling postoperative pain in our pediatric patients after CRPP of supracondylar humeral fractures. It is time to reconsider the routine prescribing of opioids and shift toward the use of non-opioid analgesia as a standard in these pediatric cases.
JBJS Deputy Editor for Social Media
Additional perspective on this study is offered in a related commentary by R. Dale Blasier, MD, FRCS(C), MBA: It’s Time to Rein in the Opioids.