Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, offers this post on 2 level-I studies in the current issue of JBJS.
In recent posts, I’ve discussed the difficulty of and dedication required in performing randomized controlled trials (RCTs) as we strive for high-quality evidence in the field of orthopaedics. Seeking to improve the level of evidence, the double-blinded RCT, in which the patients and researchers are blinded to the treatment, is an attempt to reduce potential bias and thus improve the accuracy of the findings. These studies are important to highlight as they take rigorous control and are extremely difficult to perform.
In the current issue of JBJS, there are 2 double-blinded RCTs we should review:
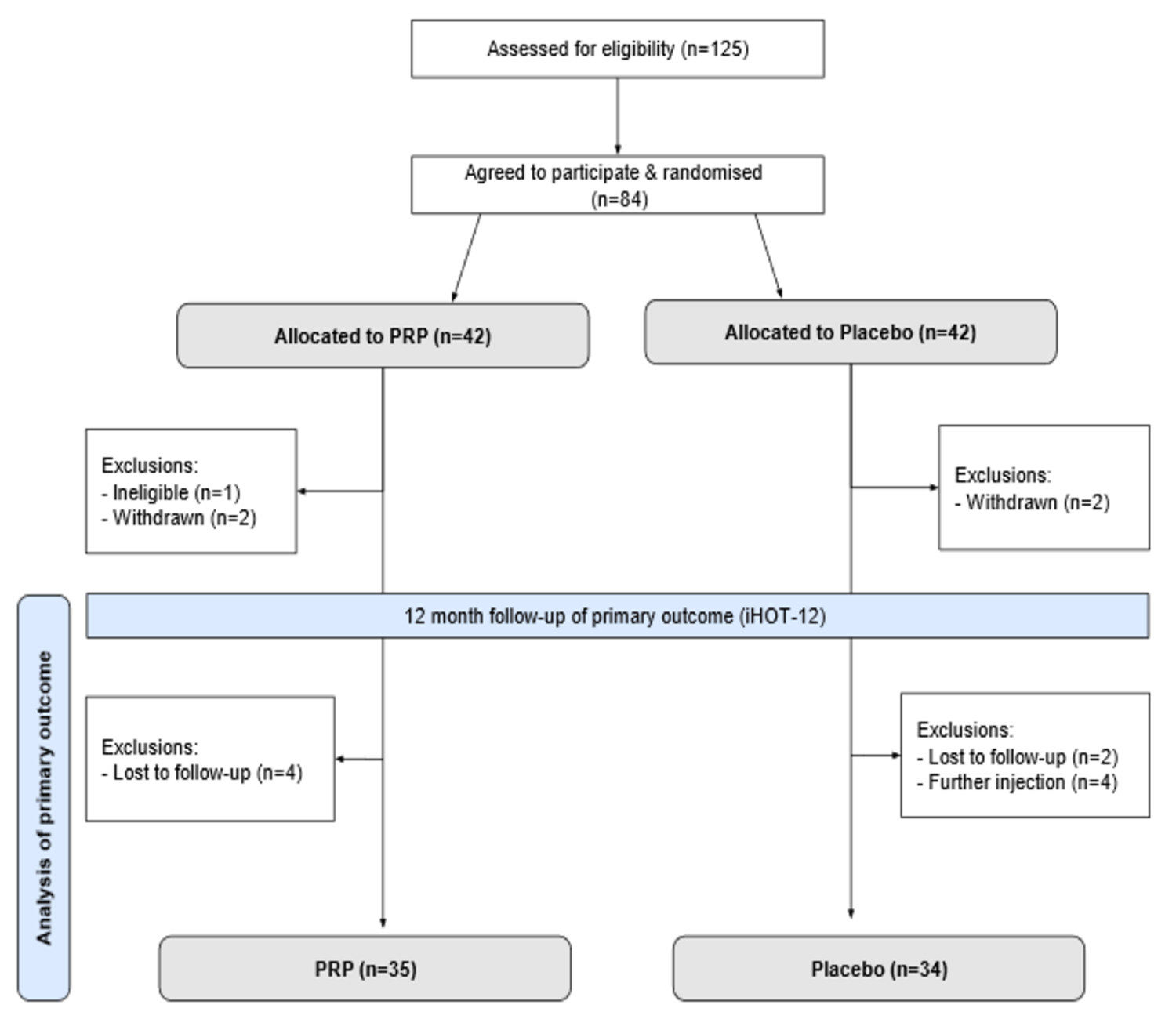
In the first study, performed in the United Kingdom, Atchia et al. investigated the efficacy of leukocyte-rich platelet-rich plasma (LR-PRP) injection compared to placebo in treating greater trochanteric pain syndrome (GTPS), a condition often characterized by hip pain and more prevalent in middle-aged women. The final analysis of this double-blinded RCT included 79 patients with chronic GTPS unresponsive to nonoperative treatments. The participants were randomized to an injection of LR-PRP versus saline before undergoing a standardized physical therapy protocol. Outcome measures included the International Hip Outcome Tool-12 (iHOT-12), visual analogue scale (VAS) for pain, the modified Harris hip score (mHHS), and the EuroQol 5-Dimensions (EQ-5D) questionnaire assessed at baseline, 3, 6, and 12 months.
The results showed no significant differences in outcomes between the LR-PRP and placebo groups at any follow-up point, although both groups experienced some improvement. The authors concluded that LR-PRP injection does not provide superior clinical outcomes compared to placebo for refractory GTPS, and they recommend against the routine use of PRP for this condition, highlighting the importance of considering the natural history of GTPS and the potential placebo effect in treatment responses. Read the full study: Efficacy of Platelet-Rich Plasma Versus Placebo for the Treatment of Greater Trochanteric Pain Syndrome. A Double-Blinded Randomized Controlled Trial
In the second study, El Ghoneimy et al. investigated the safety and efficacy of intraoperative tranexamic acid (TXA) infusion in limb-salvage surgery for malignant bone tumors in pediatric patients. Conducted at the Children Cancer Hospital in Cairo, Egypt, this RCT involved 48 participants <18 years of age, all diagnosed with a malignant bone tumor of the femur. Participants were allocated to either a TXA group receiving a loading dose followed by continuous infusion or a placebo group receiving normal saline.
The results indicated no significant difference between the groups with respect to intraoperative blood loss or transfusion requirements. However, significant differences were observed in perioperative blood loss on postoperative day 1 and at hospital discharge, as well as in total blood transfusion volumes. Importantly, there were no drug-related adverse events recorded.
The authors concluded that TXA is safe for use in this population and effectively reduces perioperative blood loss and transfusion needs, although it did not significantly impact intraoperative metrics.
Positive findings (or differences) in scientific studies are important to report, but equally important are negative findings, or studies that don’t find statistical differences, especially as we try to determine whether to augment our treatment algorithms with adjunctive (and sometimes expensive) medical treatments.
In the example of PRP for GTPS, this potentially costly orthobiologic treatment was not shown to be superior to placebo. This is important as we counsel patients in the rapidly expanding field of orthobiologics. As far as TXA in the surgical treatment of extremity cancer in pediatric patients, its use appears safe and may reduce the need for blood transfusions in these vulnerable patients, a benefit that seems to justify any added cost of the medication.
Co-author Ahmed Mohamed El Ghoneimy, MD discusses the latter investigation in an Author Insights video, found here alongside the full study. In addition, Yi Guo, MD offers further perspective in a commentary article: TXA Use in Adolescent Patients Undergoing Sarcoma Surgery
Again, I commend the author groups on their dedication to the field of orthopaedics and for striving to perform clinically sound and scientifically solid research. Such studies help to advance our efforts to achieve the highest level of evidence possible, for the best care of our patients.
JBJS Deputy Editor for Social Media