This Resident Roundup post comes from Andrew Harris, MD, who is a PGY5 resident with the Department of Orthopaedic Surgery at Johns Hopkins Hospital. Work-life
New findings in spine surgery are covered in the latest JBJS Guest Editorial What’s New in Spine Surgery. Here, we summarize the 5 most insightful

Several new papers representing a collaborative, multicenter effort to provide investigative insights into critical aspects of fracture management are now available in a special collection
A special digital collection featuring the most-read JBJS Trauma articles from 2023 to 2025 is now available. Use the following link to fill out a
Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, offers this post on 2 new studies now available at JBJS.org. As a pediatric orthopaedic surgeon
Topics of interest in foot and ankle surgery are discussed in the new JBJS Guest Editorial What’s New in Foot and Ankle Surgery. Here, we
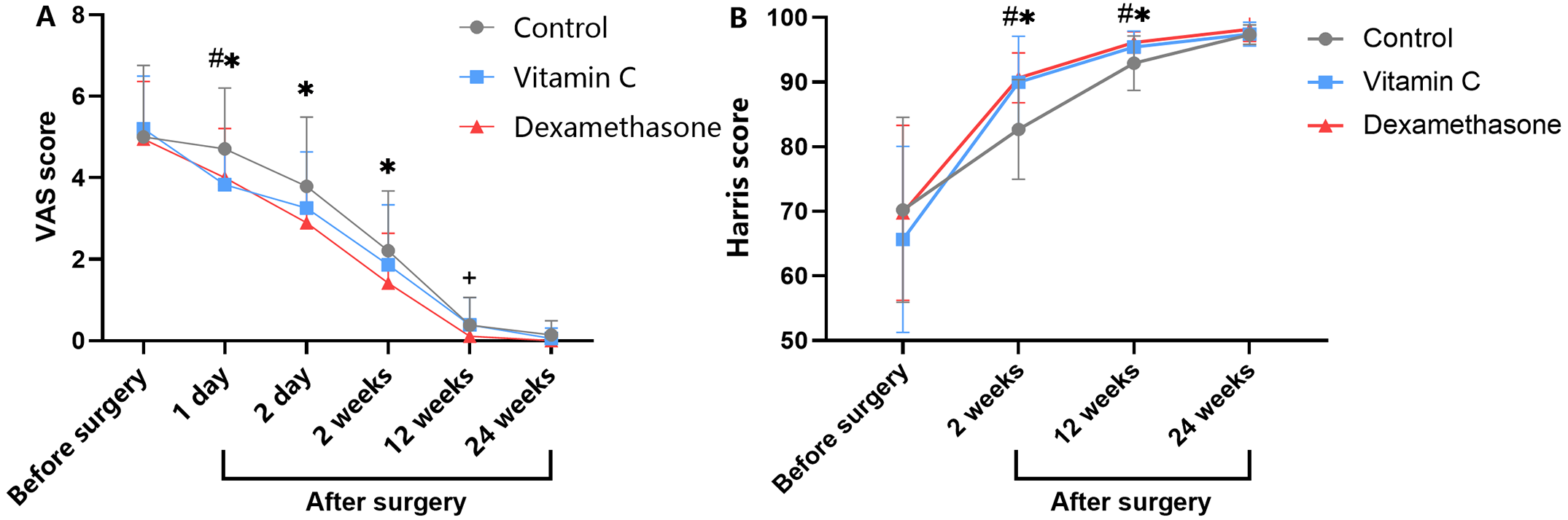
Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, highlights 2 new Level-I studies in total joint arthroplasty. Total knee arthroplasty (TKA) and total hip

Publishing high-quality evidence that drives surgical decision-making remains a key JBJS commitment. Building on this, several new article types will soon be introduced to the
Key findings in sports medicine are highlighted in the new JBJS Guest Editorial What’s New in Sports Medicine. Here, we feature the 5 most impactful
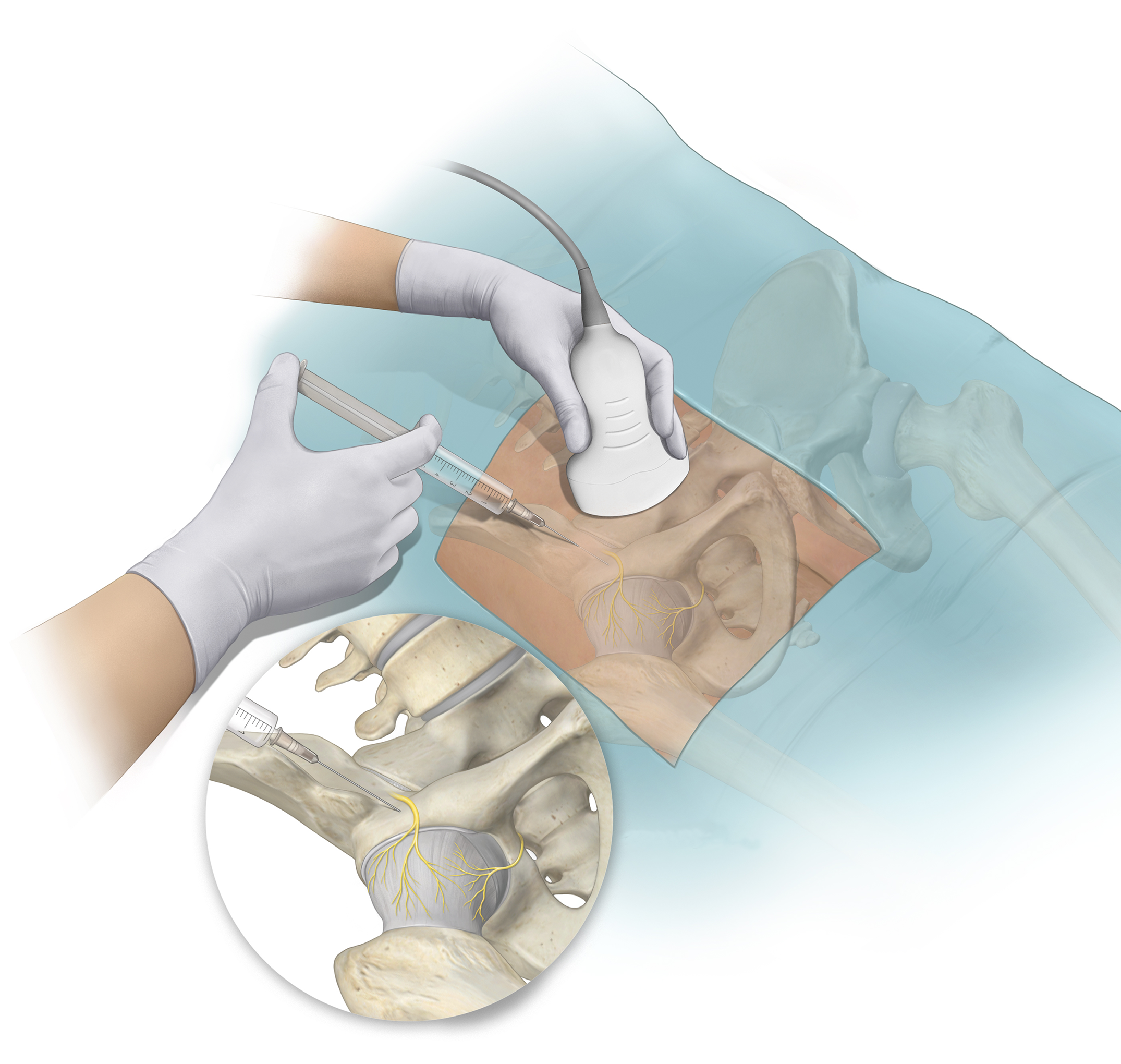
Dr. Matt Schmitz discusses 2 new RCTs investigating regional blocks in total knee and hip arthroplasty. The use of regional blocks in orthopaedic surgery has

