The recent JBJS Guest Editorial “What’s New in Osteoporosis and Fragility Fractures” provides an update on this important area of orthopaedic research. The authors review
Search Results for: atypical femoral fracture
Osteoporosis is the major contributor to the increasing incidence of fragility fractures associated with low-energy falls. The other contributor is the populous baby-boomer generation that
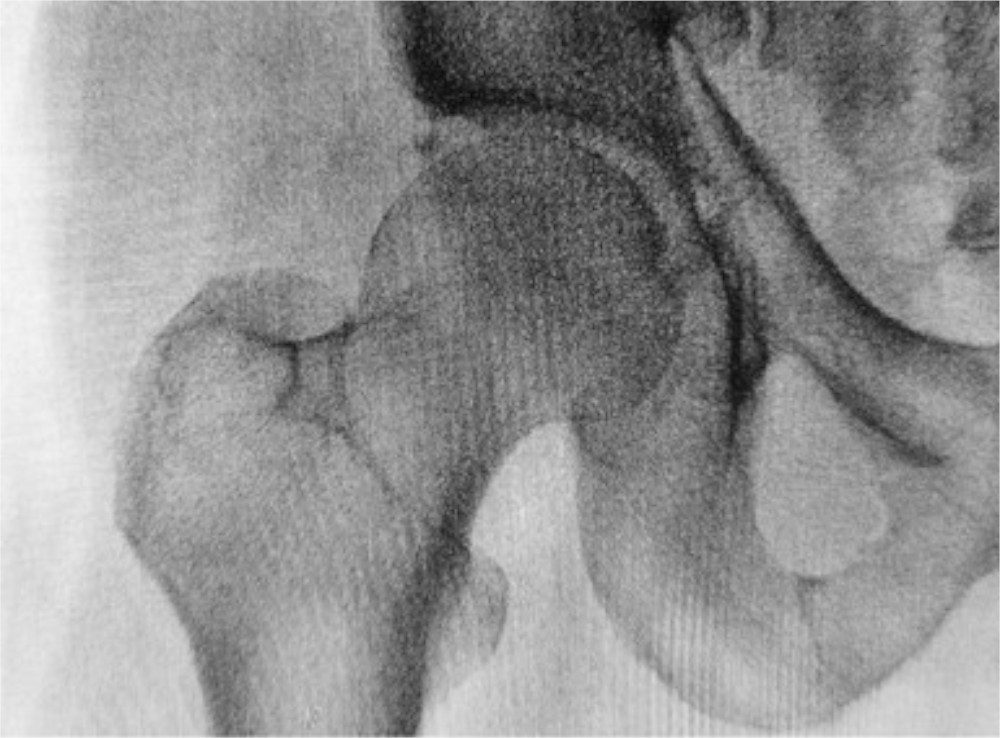
Orthopaedic journals and OrthoBuzz have devoted ample space to the apparent association between long-term bisphosphonate use and atypical femoral fractures. The latest insight into this
BMJ recently published two studies of interest to orthopaedists: After analyzing data from more than 200 cardiovascular, orthopaedic, and neurologic devices approved in both the
We posted our first “Case Connections” article about bisphosphonate-related atypical femoral fractures (AFFs) one year ago. Since then, JBJS Case Connector has published three additional case reports on
Physicians worldwide frequently prescribe bisphosphonates such as alendronate (Fosamax) and ibandronate (Boniva) to treat osteoporosis and prevent fragility fractures. Unfortunately, long-term bisphosphonate use has been
In December 1996, a group of investigators reported the results of the Fracture Intervention Trial, a randomized controlled trial that compared the effect of alendronate