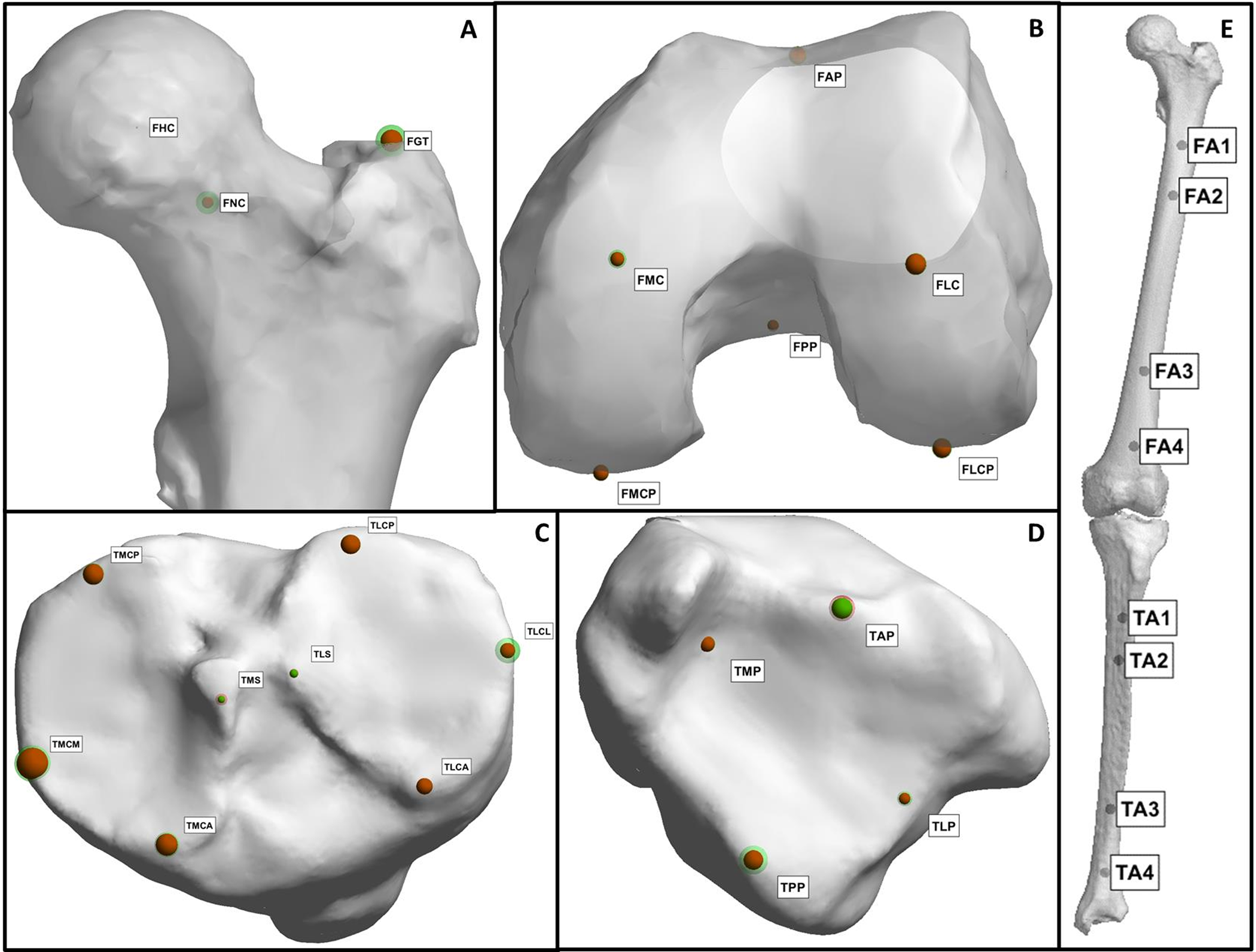
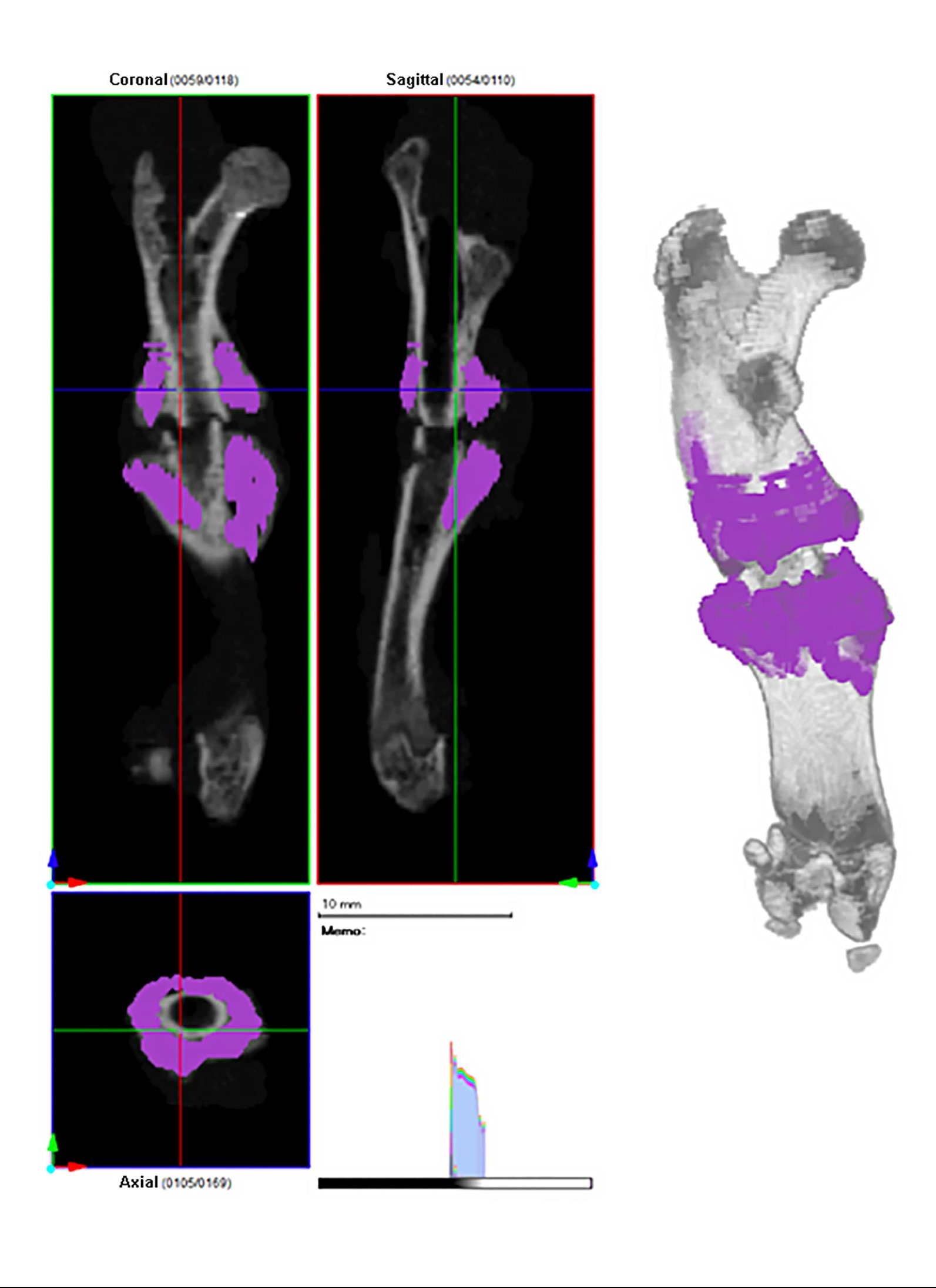
Editor-in-Chief Dr. Marc Swiontkowski discusses a new study from The Netherlands that presents a fully automatic method for assessing lower-limb alignment from computed tomography (CT)
Category: Basic Science

Recent groundbreaking studies are summarized in the JBJS Guest Editorial “What’s New in Musculoskeletal Basic Science,” now available at JBJS.org. Here, we spotlight the 5
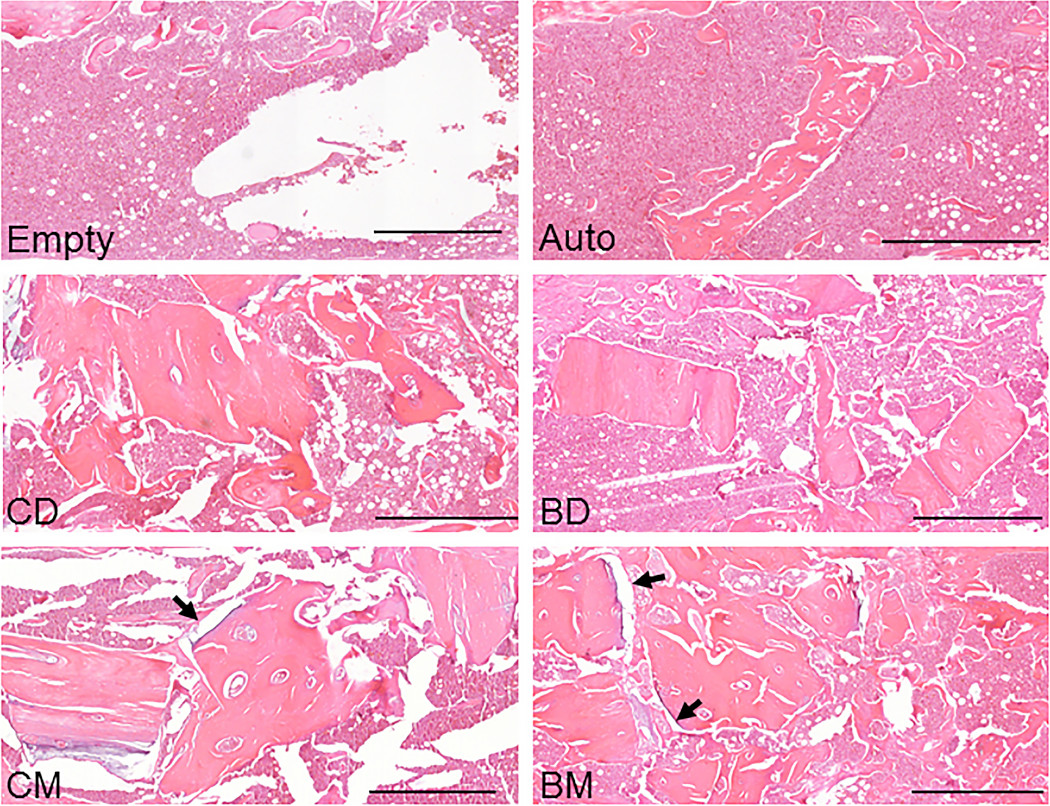
The findings of a new animal study suggest that bisphosphonate treatment in donors may indeed be relevant when mineralized allografts are used in orthopaedic procedures.
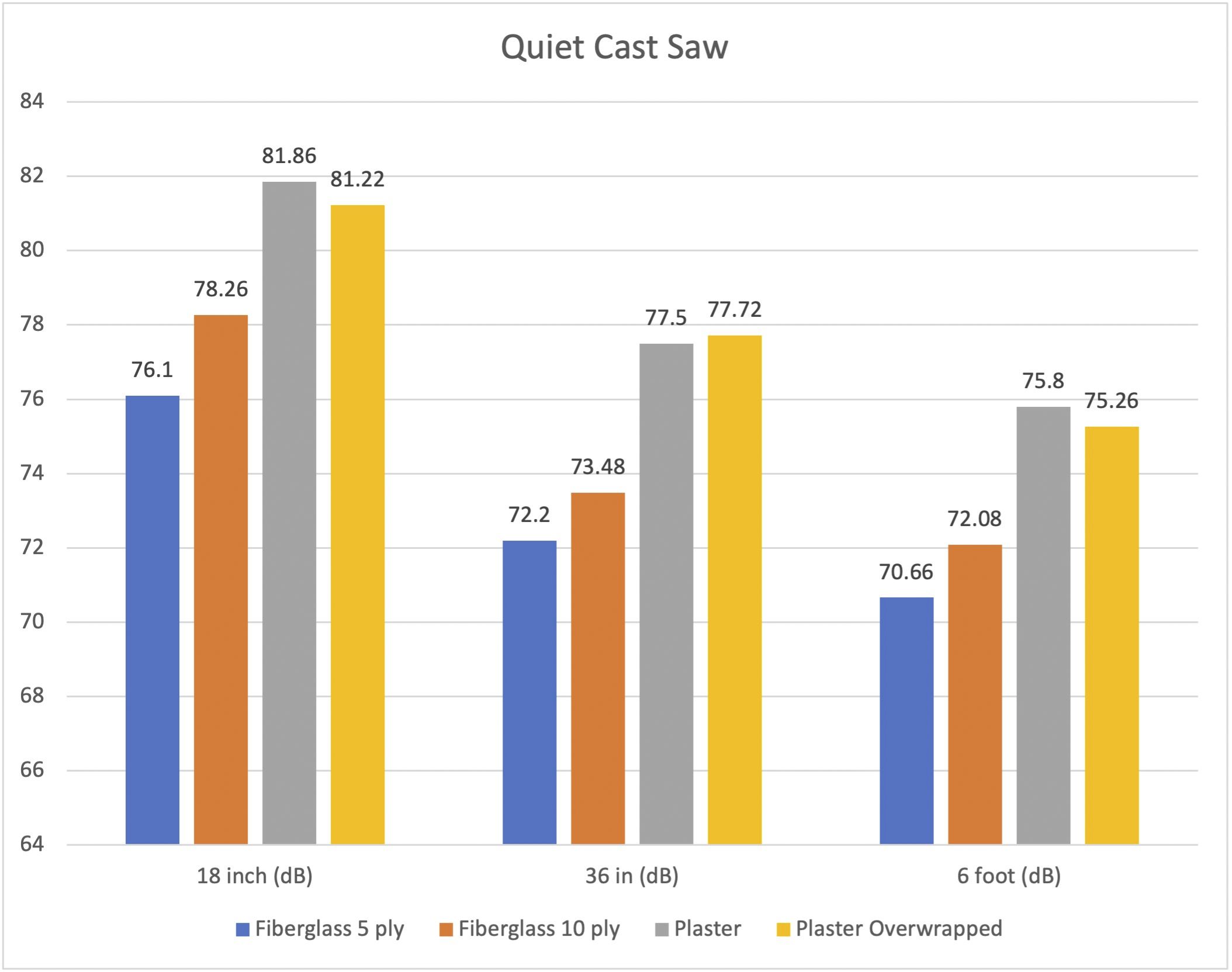
A new study in JBJS examines noise levels during cast removal. In this post, JBJS Editor-in-Chief Dr. Marc Swiontkowski reflects on the importance of research
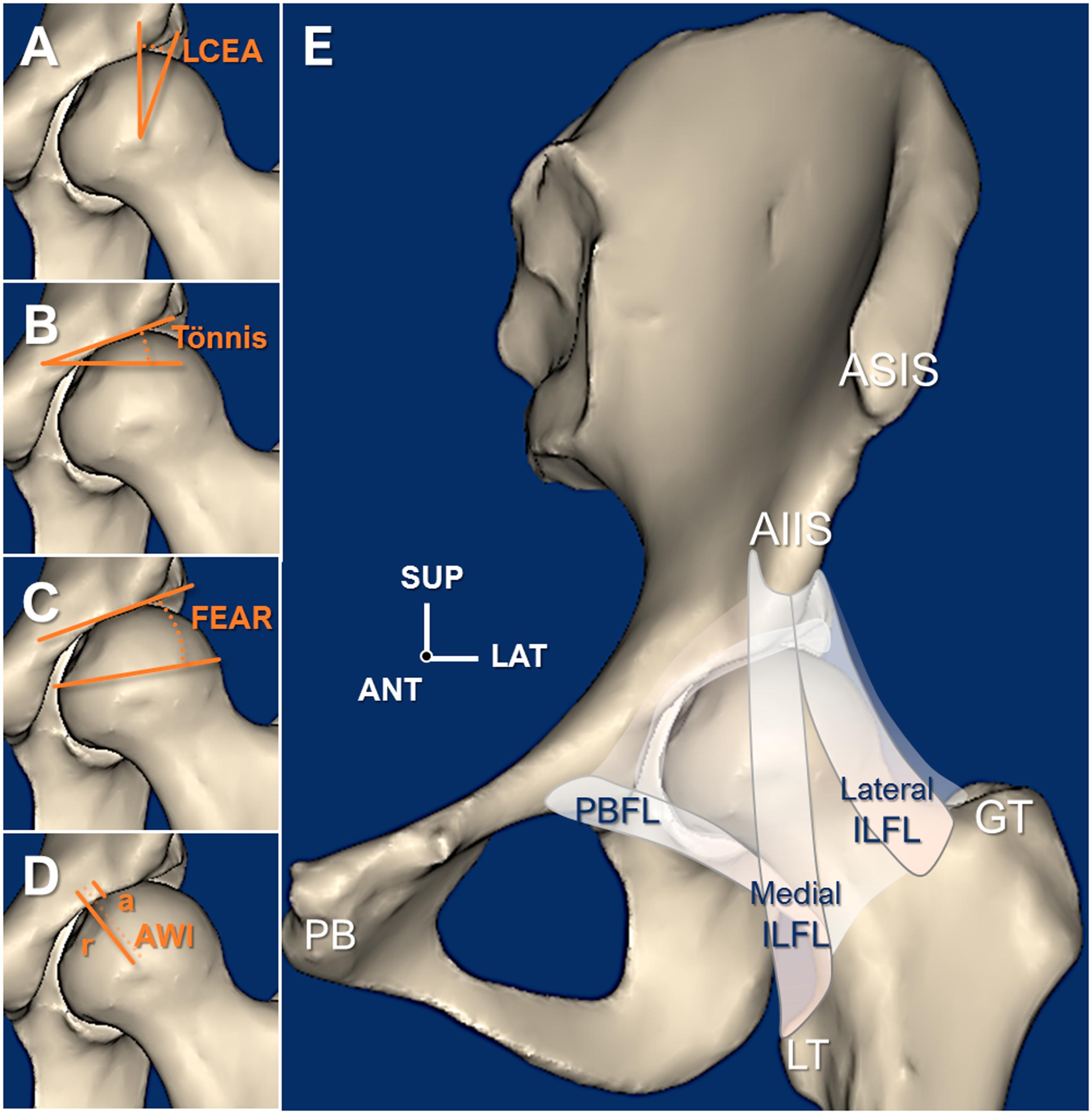
Hip dysplasia is known to cause early joint deterioration, instability, and pain. It can be corrected with the Bernese periacetabular osteotomy (PAO). However, stability of
Surgical site infections (SSIs) can be devastating complications for orthopaedic patients. A new study published in Nature advances our understanding of drug-resistant pathogens that can

Every month, JBJS publishes a review of the most pertinent and impactful studies from the orthopaedic literature during the previous year in 14 subspecialty areas. This month, co-author
An extensive body of clinical and basic science research has confirmed that cigarette smoking negatively impacts bone healing. Newer products, such as electronic cigarettes (e-cigarettes) and heated tobacco products (HTPs), are often described as safer alternatives to traditional
Periprosthetic joint infection (PJI) remains a challenging complication in orthopaedics. These infections are often related to low-virulence organisms, and the search for reliable diagnostic tests continues to be paramount. Joint aspiration has been a starting point for
The incorporation of antibiotics within polymethylmethacrylate (PMMA) has been widely used over recent decades for managing infection following skeletal trauma. Early research helped to