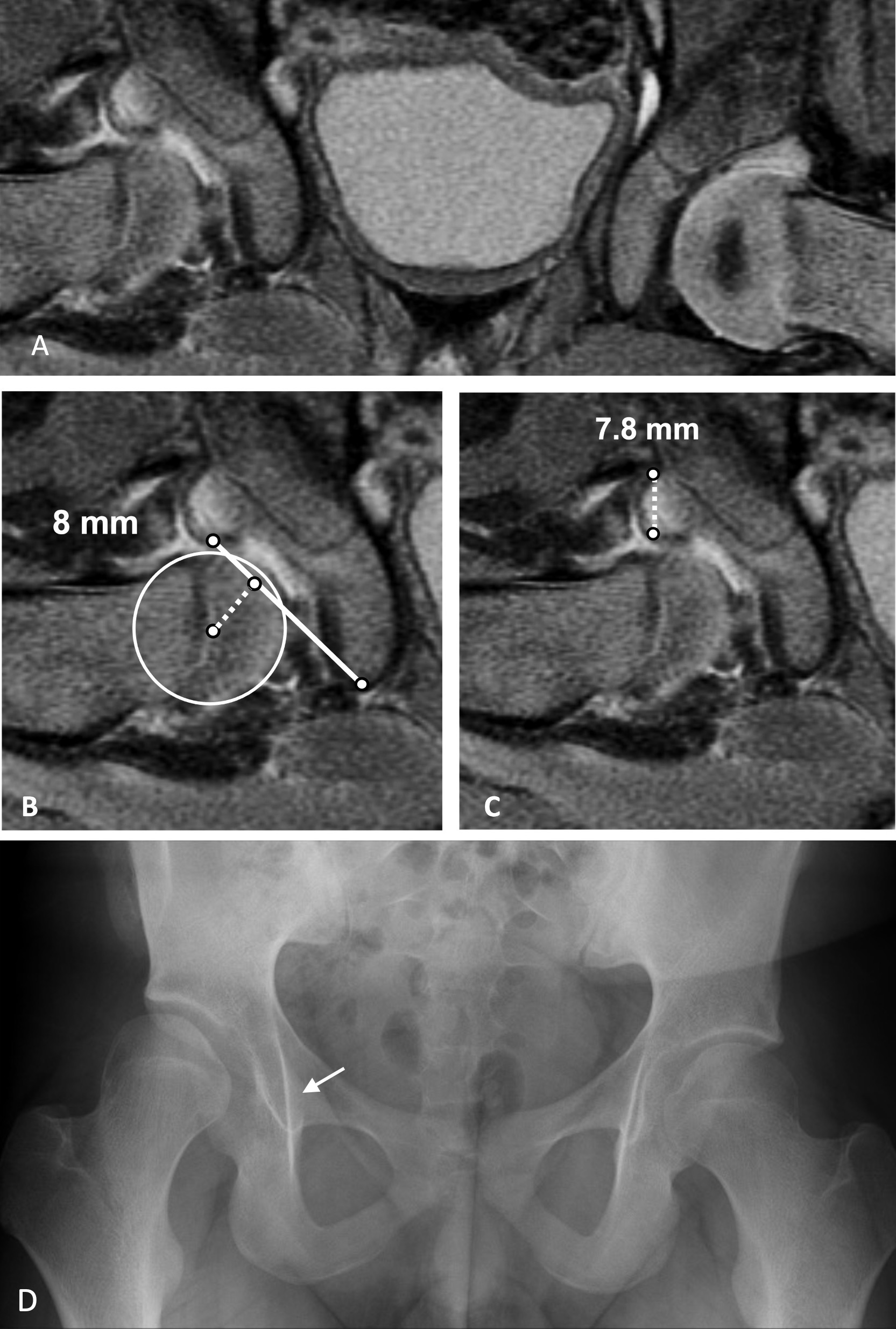
JBJS Deputy Editor for Social Media Dr. Matt Schmitz discusses a new study assessing the use of post-reduction MRI measurements to help predict residual acetabular
Category: Hip
Swimming is often recommended after total hip or knee arthroplasty. Previous research has shown that aquatic exercises reduce joint loads and enhance muscle strength in
JBJS Editor-in-Chief Dr. Marc Swiontkowski reflects on a new study examining changes in skeletal muscle mass following femoral fragility fracture. Since the early 20th century,
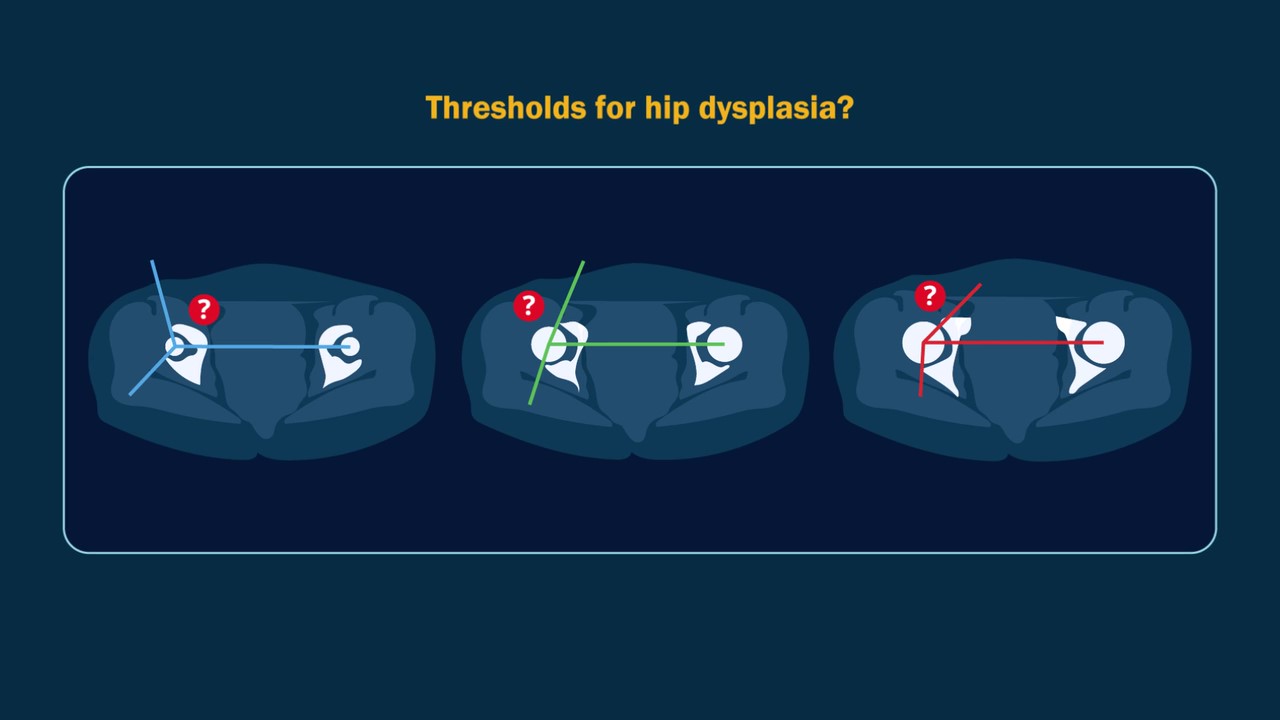
A video abstract is available with the new study by Verhaegen et al. in JBJS: Acetabular Sector Angles in Asymptomatic and Dysplastic Hips: Defining Dysplasia
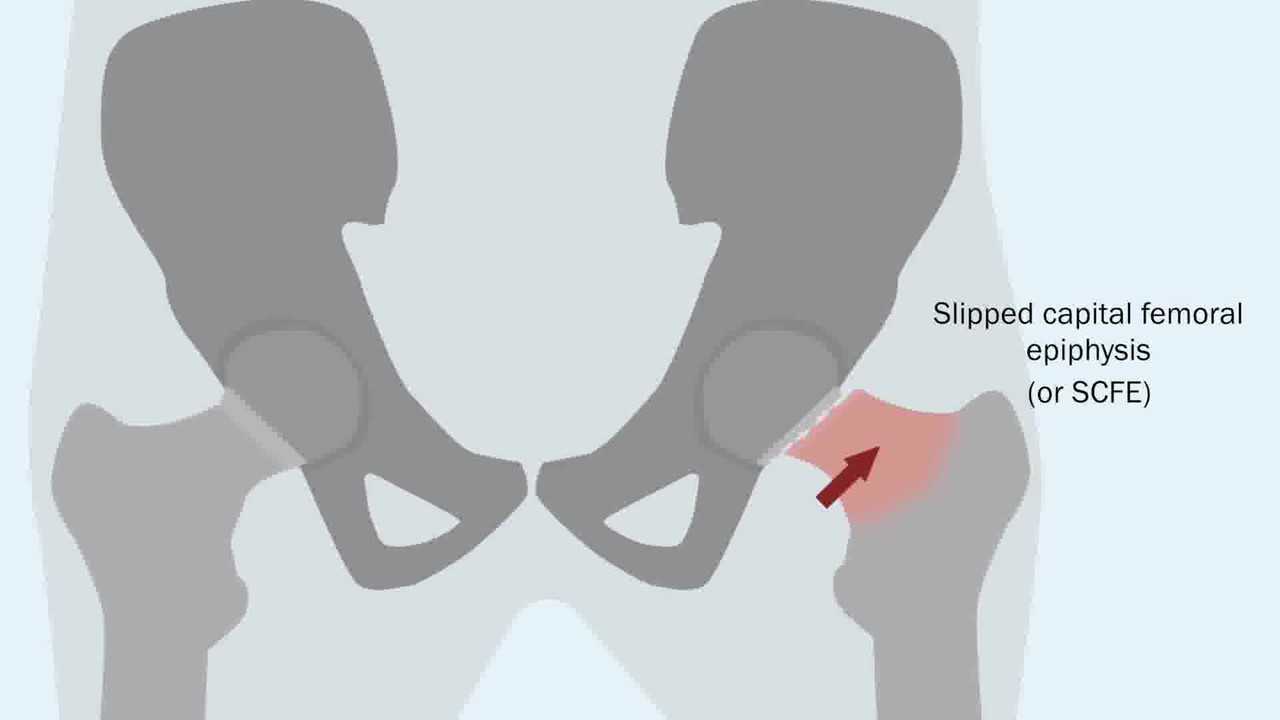
In this post, Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, discusses a new study examining the impact of social determinants of health on
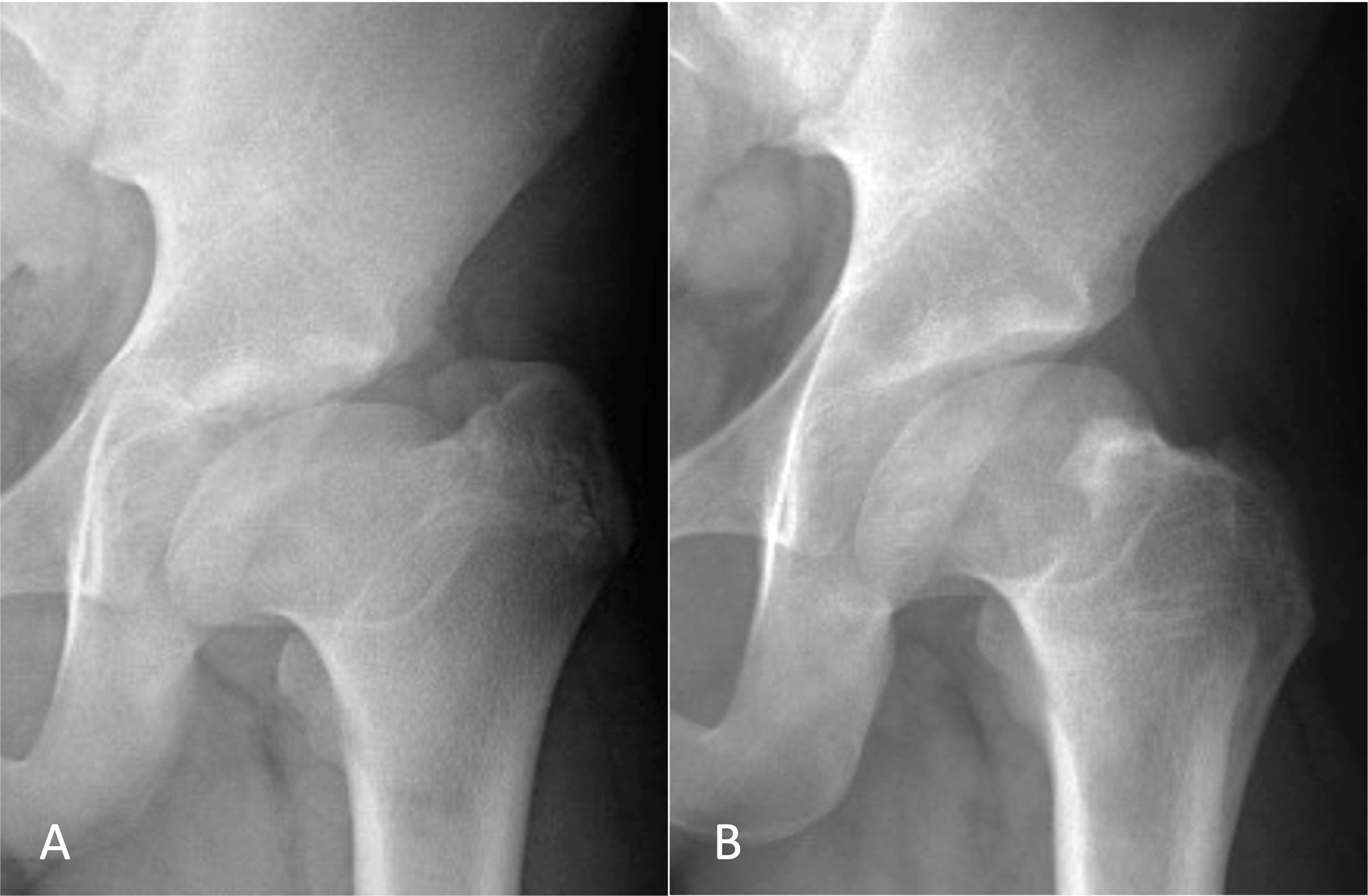
In a new study in JBJS, Novais et al. note the challenge of treating patients with symptomatic hips after healed Legg-Calvé-Perthes disease (LCPD) given the
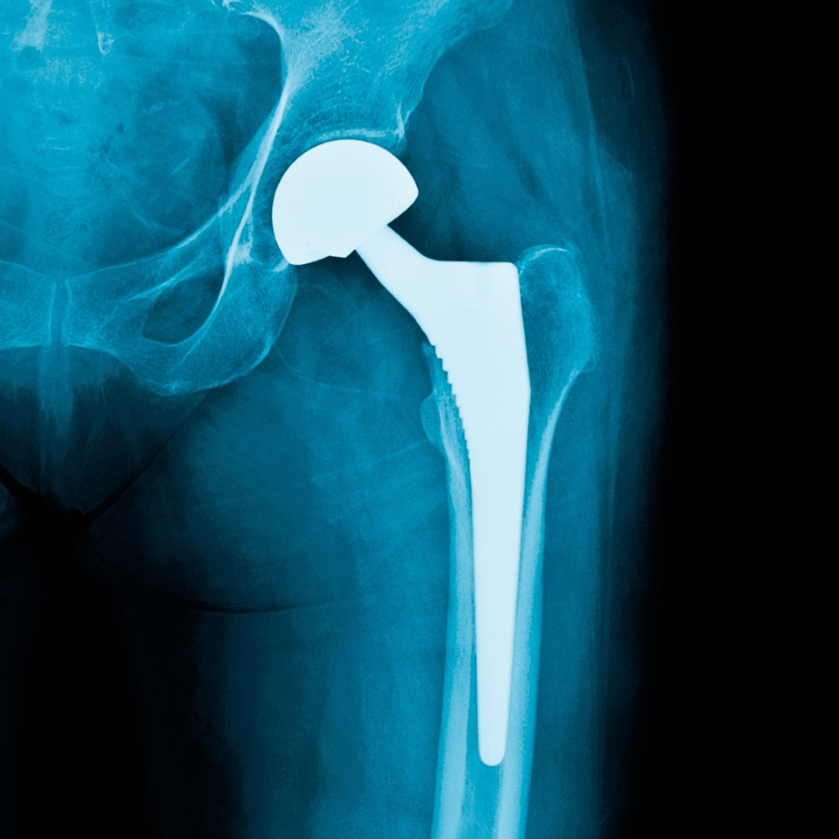
Periprosthetic joint infection, venous thromboembolism prevention, and implant cost-utility are among the focuses of the new JBJS Guest Editorial What’s New in Hip Surgery. Here,
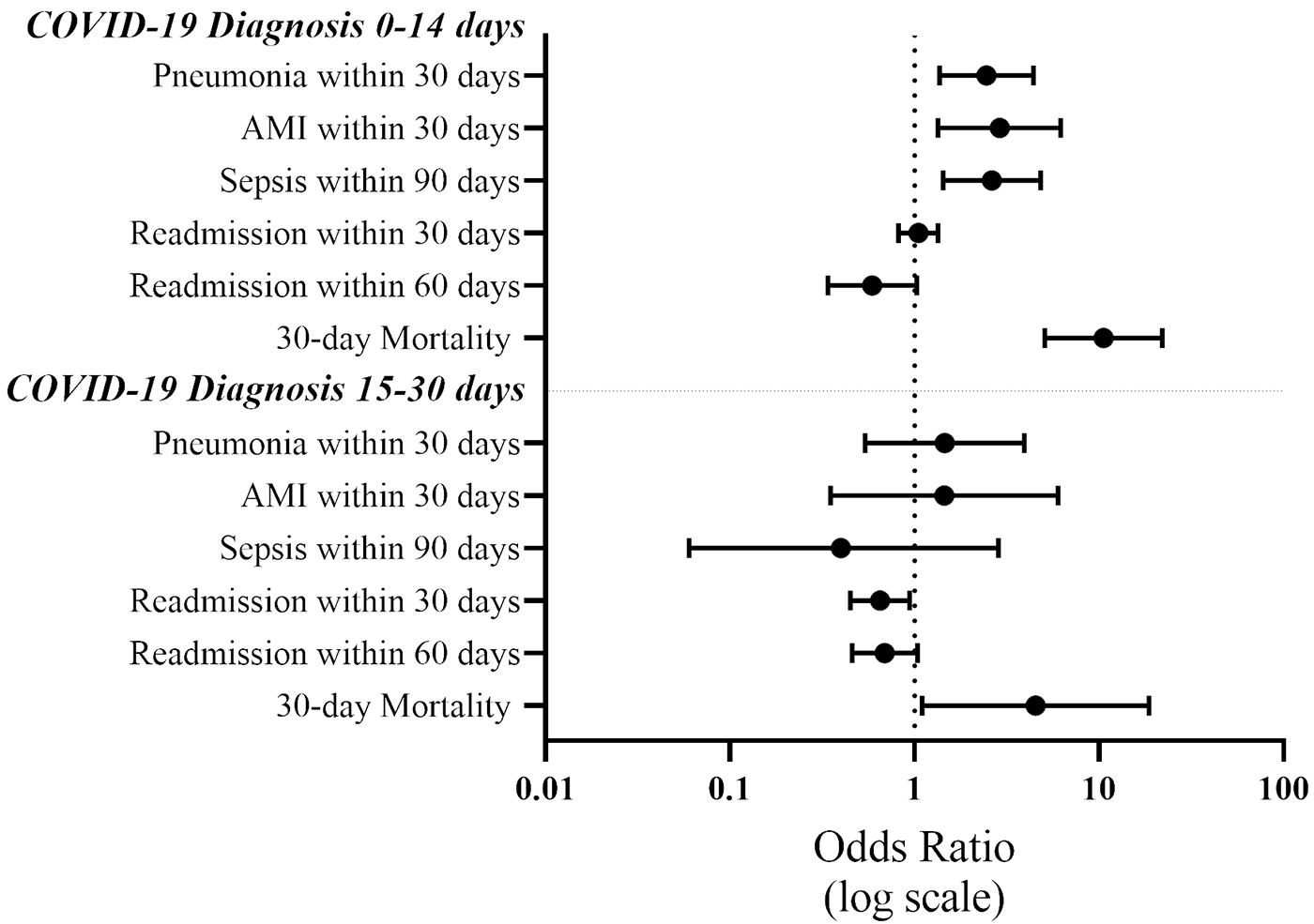
JBJS Deputy Editor for Social Media Dr. Matt Schmitz reflects on a new study evaluating postoperative complications and mortality in patients who had a recent
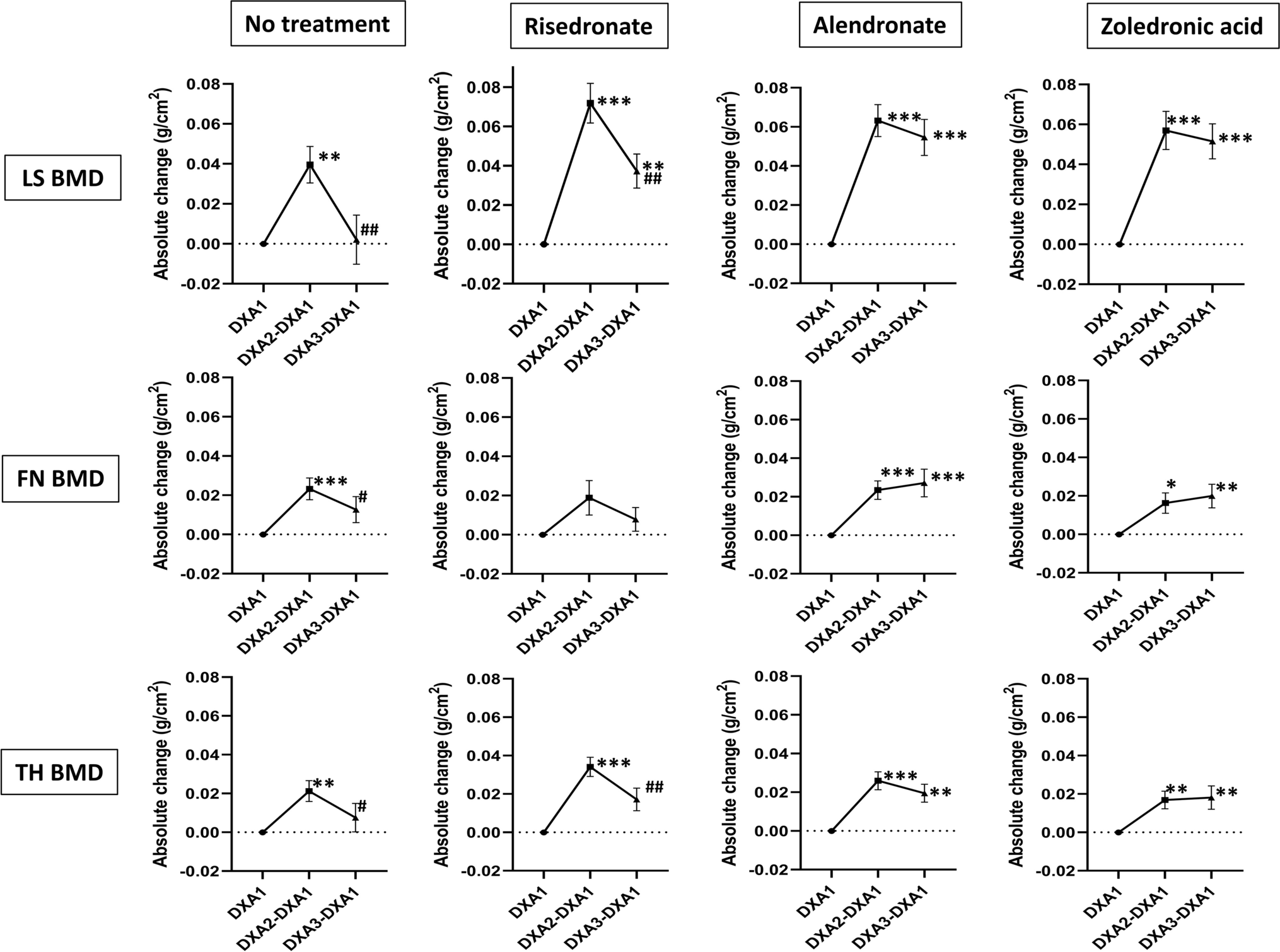
Vitamin D supplementation, fracture risk, and osteoporosis drug therapies are among several topics explored in recent studies featured in the new JBJS Guest Editorial: What’s
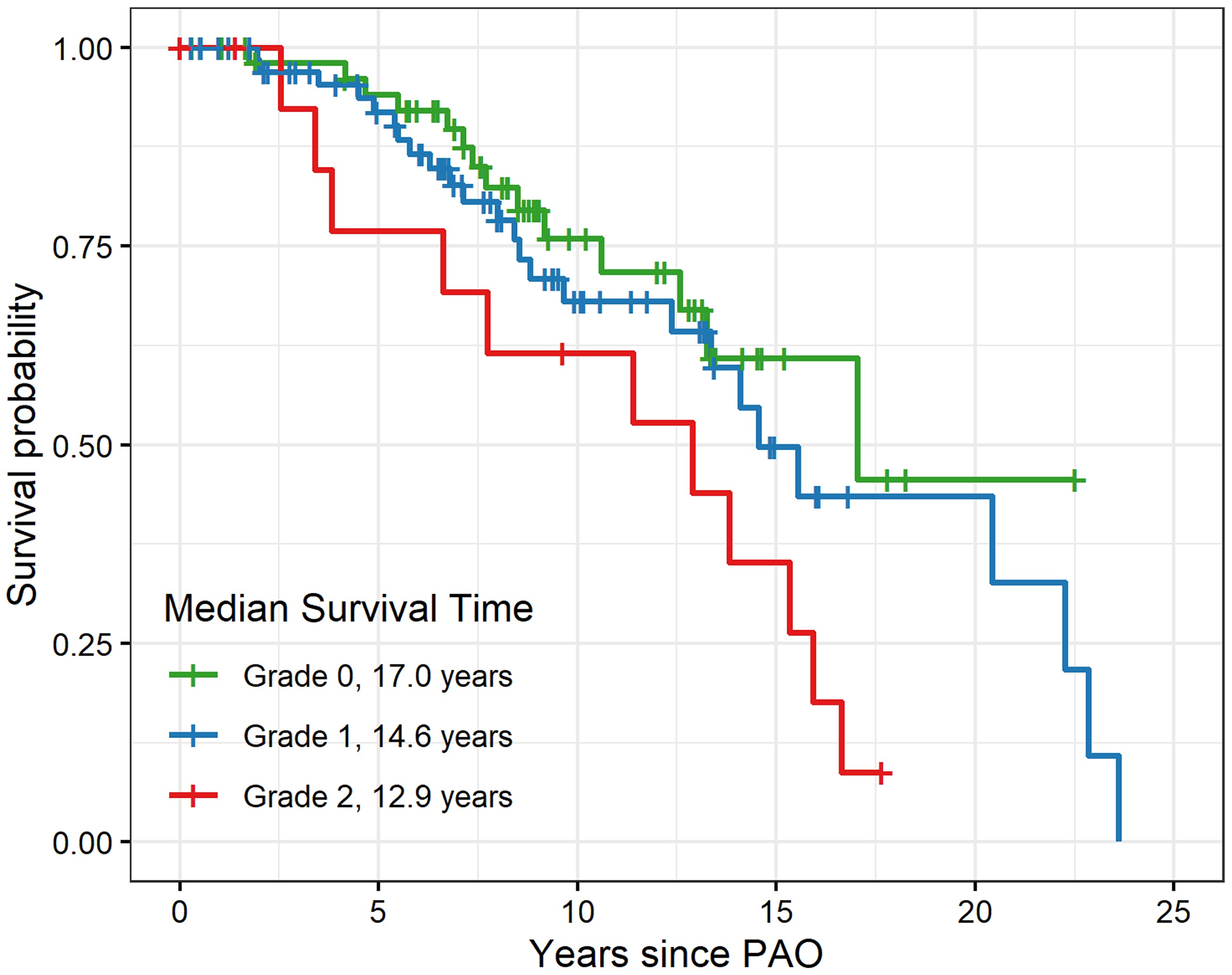
In this post, Dr. Matt Schmitz, JBJS Deputy Editor for Social Media, discusses a new study that evaluates periacetabular osteotomy for symptomatic acetabular dysplasia in