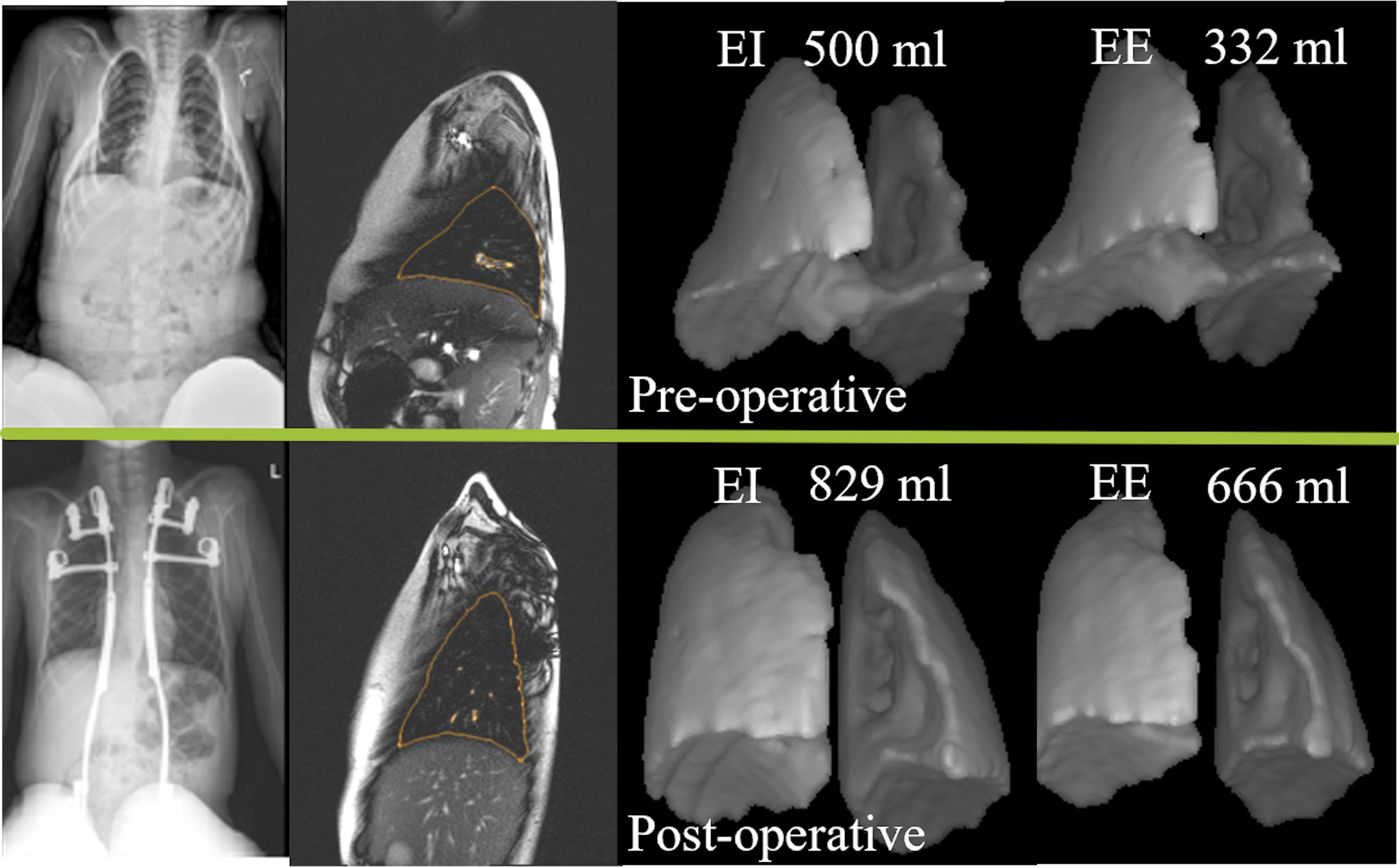
Notable findings in pediatrics regarding the treatment of fractures, infection, early-onset scoliosis, and more are presented in the new JBJS Guest Editorial What’s New in
Category: Infection
Findings from high-level and award-winning studies on topics such as unicompartmental and total knee arthroplasty (TKA), among others, are featured in the new JBJS Guest
Periprosthetic joint infection, venous thromboembolism prevention, and implant cost-utility are among the focuses of the new JBJS Guest Editorial What’s New in Hip Surgery. Here,

An international team of musculoskeletal oncology experts have explored several important questions regarding the surgical treatment of patients through secondary analyses of data from the
The new JBJS Guest Editorial “What’s New in Musculoskeletal Infection” presents findings related to chronic periprosthetic joint infection, oral antibiotic prophylaxis, and more. Here, we
The relationship between surgical site infection and preoperative corticosteroid injection for various procedures, findings on the long-term effectiveness of corticosteroid injection versus carpal tunnel release
Multiple award-winning studies—on topics such as extended antibiotic prophylaxis after total joint arthroplasty and metal debris in the knee joint following arthroplasty with nickel-free components—are
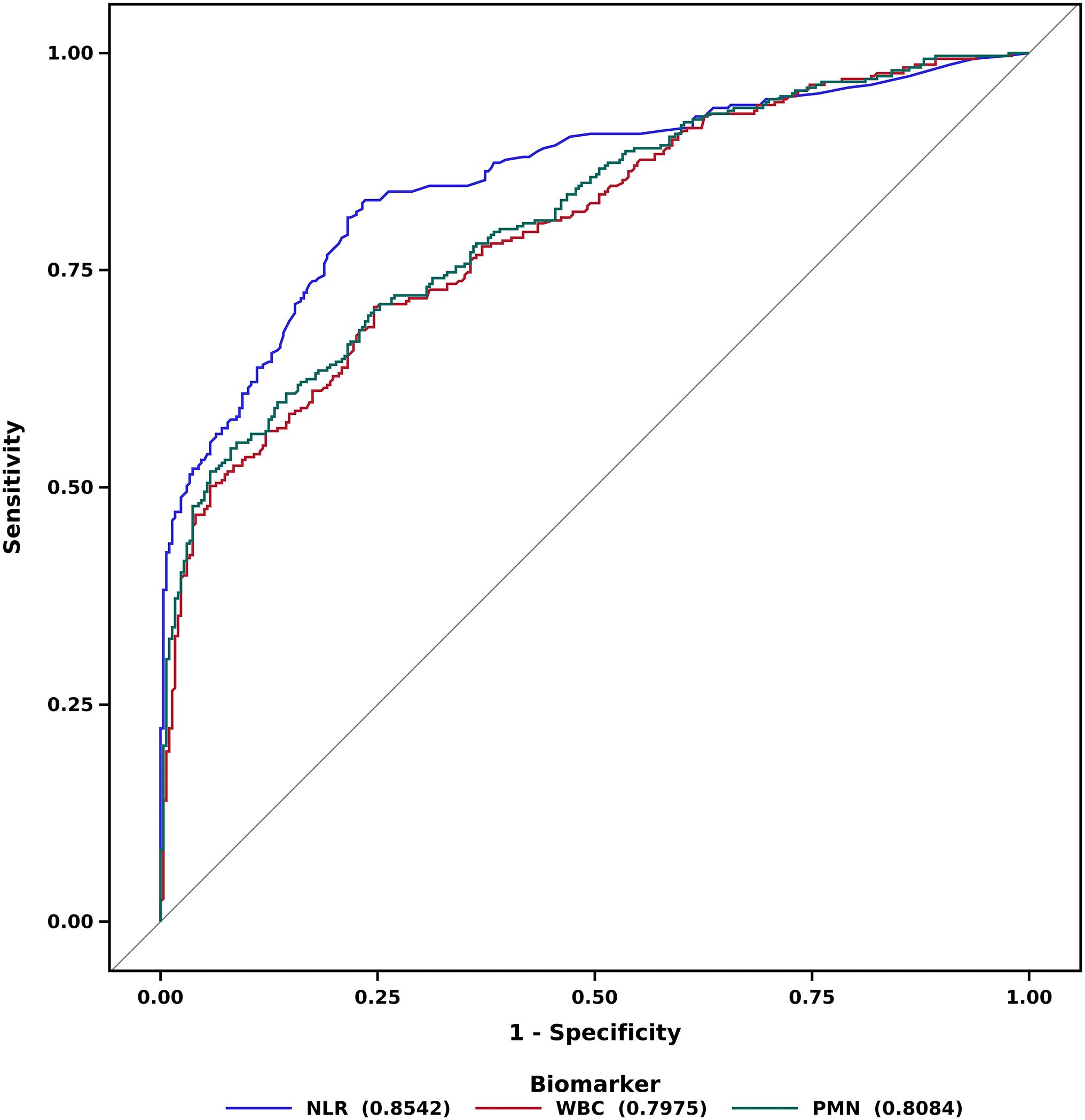
There are some orthopaedic conditions for which a delay in treatment of even a few hours can lead to poor outcomes. Septic arthritis (SA) is
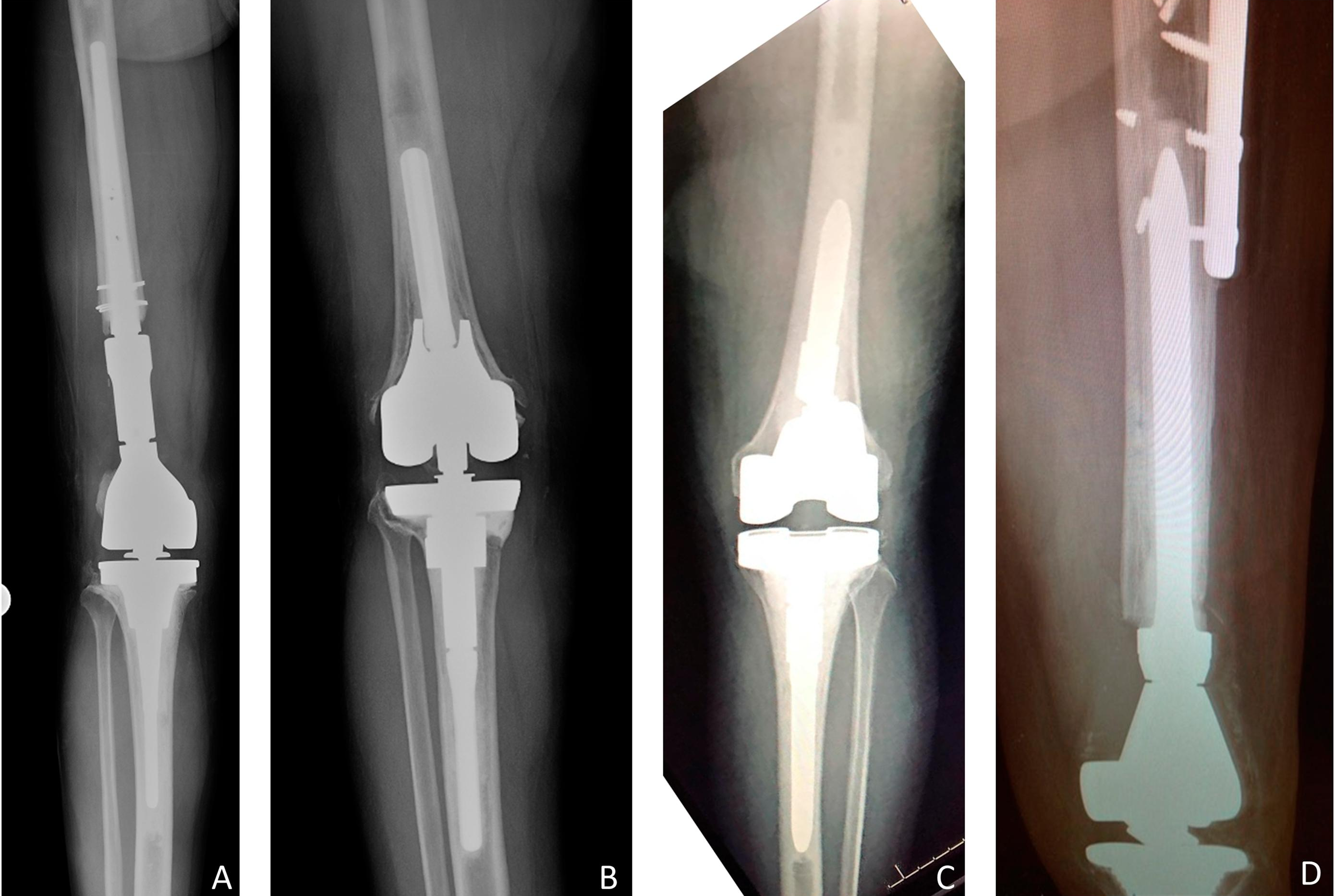
Recent studies on periprosthetic joint infection (PJI) and other topics are presented in the JBJS Guest Editorial “What’s New in Musculoskeletal Infection.” Here, we spotlight
Surgical site infections (SSIs) can be devastating complications for orthopaedic patients. A new study published in Nature advances our understanding of drug-resistant pathogens that can