The recent JBJS Guest Editorial “What’s New in Osteoporosis and Fragility Fractures” provides an update on this important area of orthopaedic research. The authors review
Tag: alendronate
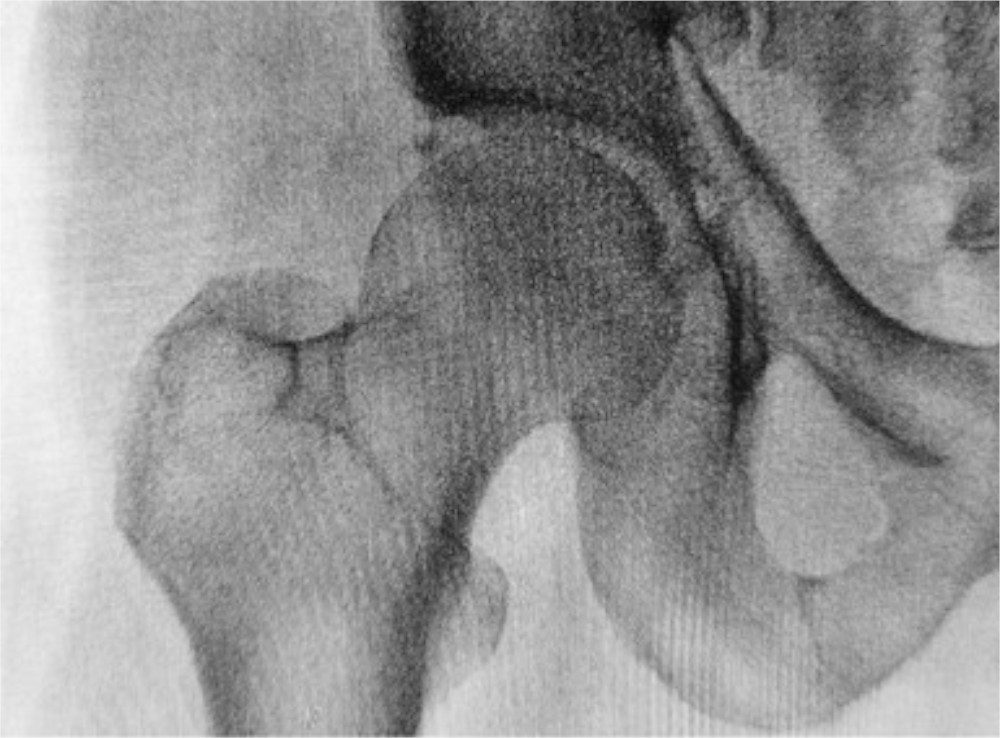
Physicians worldwide frequently prescribe bisphosphonates such as alendronate (Fosamax) and ibandronate (Boniva) to treat osteoporosis and prevent fragility fractures. Unfortunately, long-term bisphosphonate use has been
A recent study in the Journal of Clinical Endocrinology & Metabolism found that approximately one out of 200 Taiwanese who used oral alendronate long term