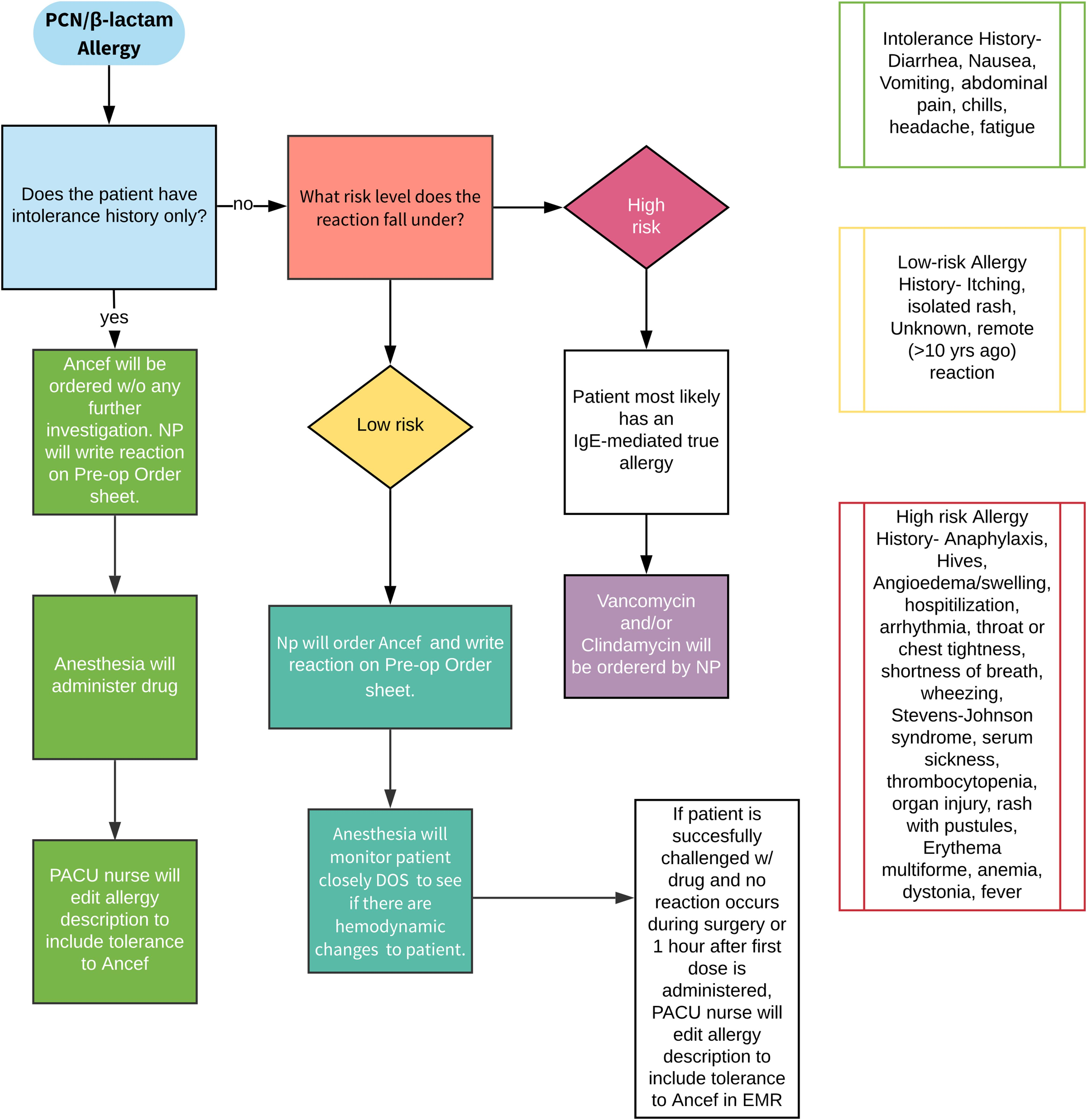
All orthopaedic surgeons must carefully decide on which prophylactic antibiotic to prescribe when a patient or a young patient’s parent reports an allergy to penicillin.
Tag: antibiotic
The incorporation of antibiotics within polymethylmethacrylate (PMMA) has been widely used over recent decades for managing infection following skeletal trauma. Early research helped to
We recently celebrated Veteran’s Day with the annual tradition of rightfully honoring the men and women who have served in the Armed Forces. After their
Antibiotics are an integral part of infection prophylaxis in orthopaedic surgery, and tourniquets are widely used during many of those same surgeries. The timing of
Surgeons performing revision shoulder arthroplasty typically order postoperative antibiotics to be administered while they wait for results from intraoperative cultures. Based on their index of
The rate of adoption of knowledge gleaned from multiple well-done randomized clinical trials into medical practice is disappointingly slow. This has been well-documented in cardiovascular
A study by Miller et al. in the February 20, 2019 issue of JBJS provides preclinical proof of concept that antibiotic-loaded coatings on orthopaedic implants
OrthoBuzz occasionally receives posts from guest bloggers. In response to a recent New England Journal of Medicine study, the following commentary comes from Daniel Leas, MD and Joseph R.
When it comes to preventing infections associated with orthopaedic procedures, the question of which antibiotic to use is only one of several concerns. How and
The number of articles published each year in orthopaedics that evaluate infections seems to approach, if not exceed, 1,000. Yet, despite all of these publications,