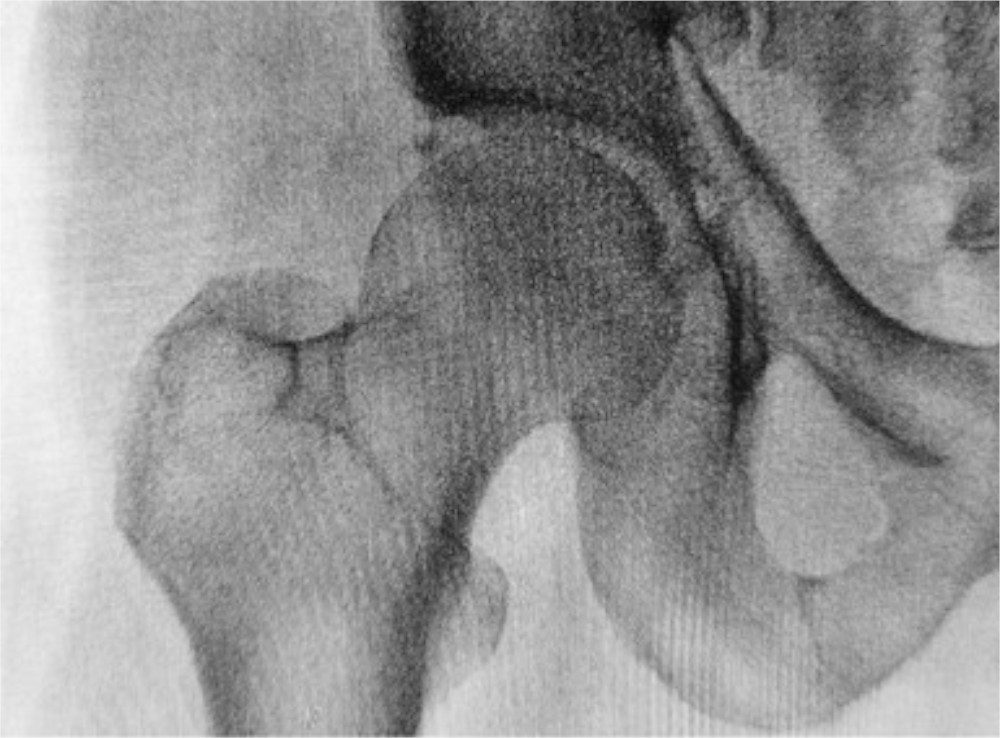
The recent JBJS Guest Editorial “What’s New in Osteoporosis and Fragility Fractures” provides an update on this important area of orthopaedic research. The authors review
Tag: atypical femoral fracture
Osteoporosis is the major contributor to the increasing incidence of fragility fractures associated with low-energy falls. The other contributor is the populous baby-boomer generation that
Orthopaedic journals and OrthoBuzz have devoted ample space to the apparent association between long-term bisphosphonate use and atypical femoral fractures. The latest insight into this
BMJ recently published two studies of interest to orthopaedists: After analyzing data from more than 200 cardiovascular, orthopaedic, and neurologic devices approved in both the