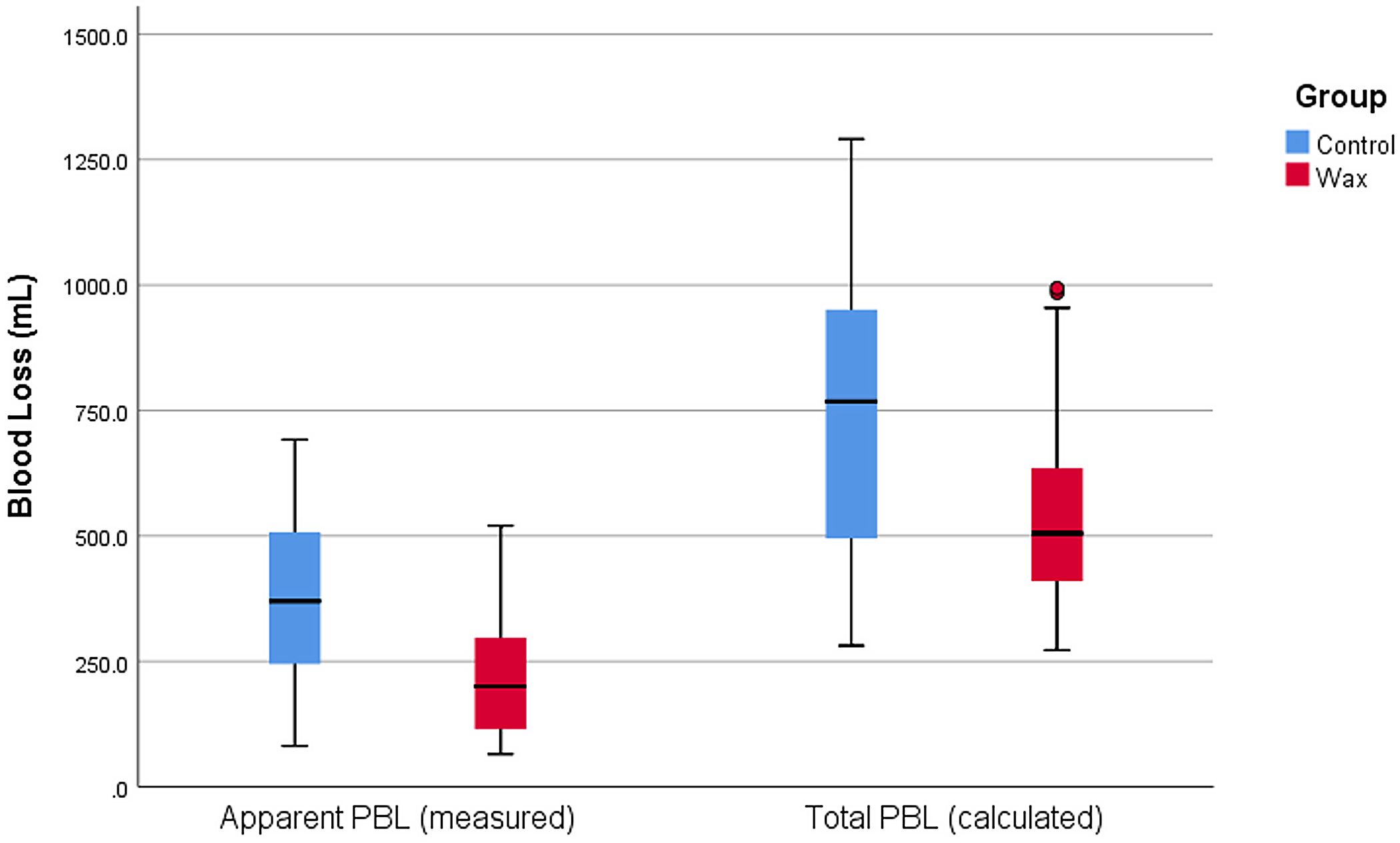
A number of measures have been suggested for reducing perioperative blood loss (PBL) in total hip arthroplasty (THA). A new randomized clinical trial in JBJS
Tag: blood loss
The recent orthopaedic literature, including a 2017 JBJS study, provides substantial evidence that oral and intravenous tranexamic acid (TXA) are equivalent in their effectiveness at
The evidence favoring tranexamic acid (TXA) for reducing surgical blood loss is ample and growing, but until now robust data were sparse regarding its efficacy
Minimizing perioperative blood loss during total knee arthroplasty (TKA) helps curtail the risks and costs of allogeneic blood transfusions. Currently, the most popular pharmacological approach