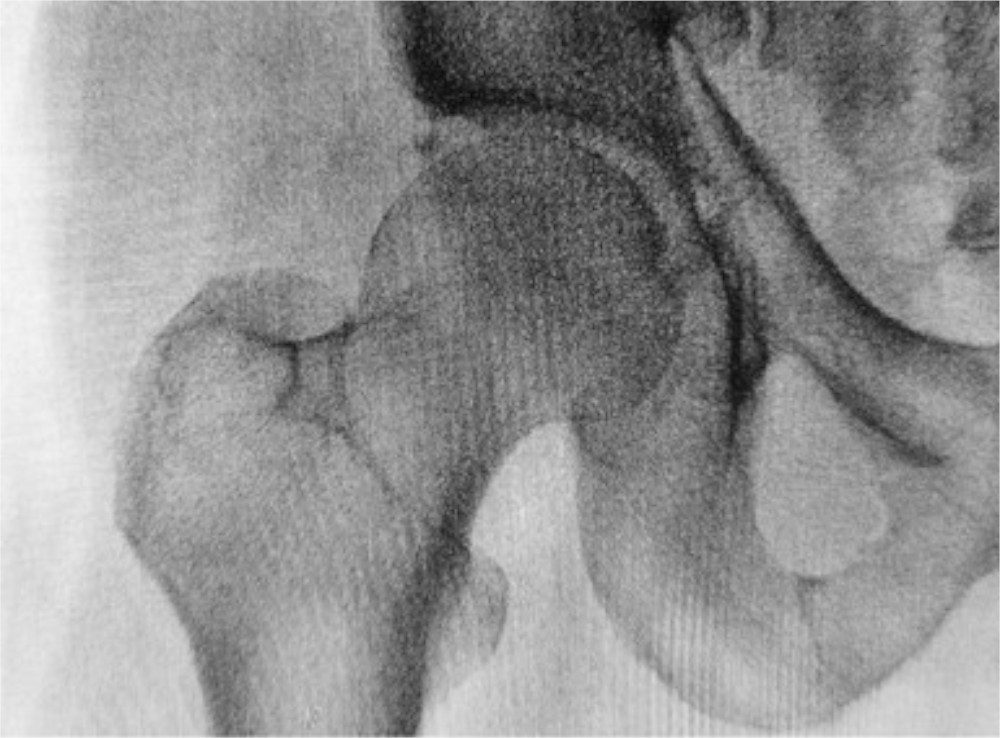
The recent JBJS Guest Editorial “What’s New in Osteoporosis and Fragility Fractures” provides an update on this important area of orthopaedic research. The authors review
Tag: fracture healing

Every month, JBJS publishes a review of the most pertinent and impactful studies from the orthopaedic literature during the previous year in 14 subspecialty areas. This month, co-author
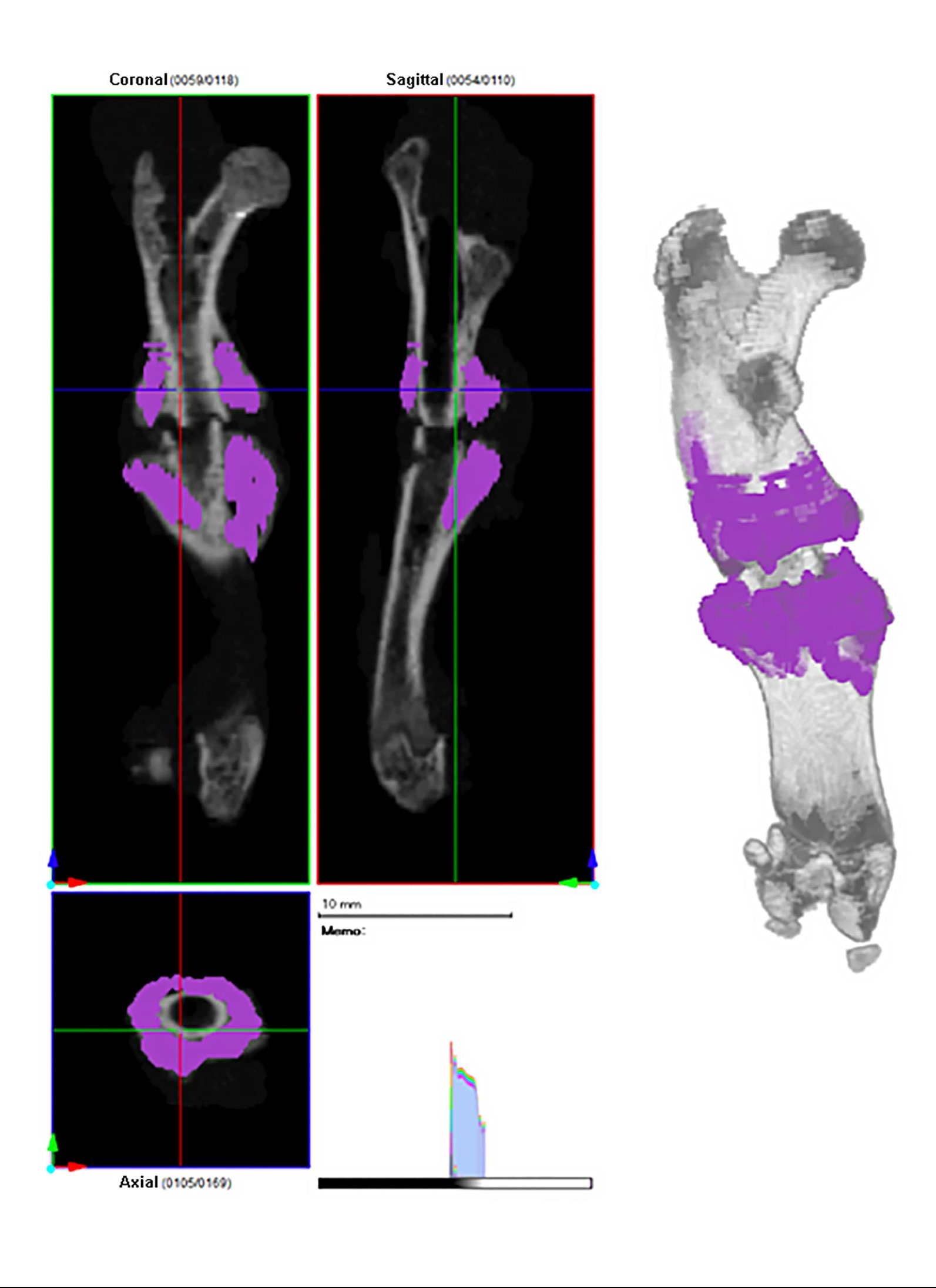
An extensive body of clinical and basic science research has confirmed that cigarette smoking negatively impacts bone healing. Newer products, such as electronic cigarettes (e-cigarettes) and heated tobacco products (HTPs), are often described as safer alternatives to traditional
Mechanical factors undoubtedly play a role in the rate and quality of fracture healing. For example, the seminal work on fracture strain by the late
In our ongoing attempt to identify pharmacologic interventions that improve fracture healing, the sclerostin inhibitor romosozumab is a logical candidate, as it has been shown
This post comes from Fred Nelson, MD, an orthopaedic surgeon in the Department of Orthopedics at Henry Ford Hospital and a clinical associate professor at
This post comes from Fred Nelson, MD, an orthopaedic surgeon in the Department of Orthopedics at Henry Ford Hospital and a clinical associate professor at
OrthoBuzz occasionally receives posts from guest bloggers. This guest post comes from E. Scott Paxton, MD, in response to a recent “Rapid Recommendation” in The BMJ.
OrthoBuzz regularly brings you a current commentary on a “classic” article from The Journal of Bone & Joint Surgery. These articles have been selected by the
Researchers at Vanderbilt University Medical Center have concluded that fibrin, a protein involved in blood clotting and found abundantly around the site of new bone