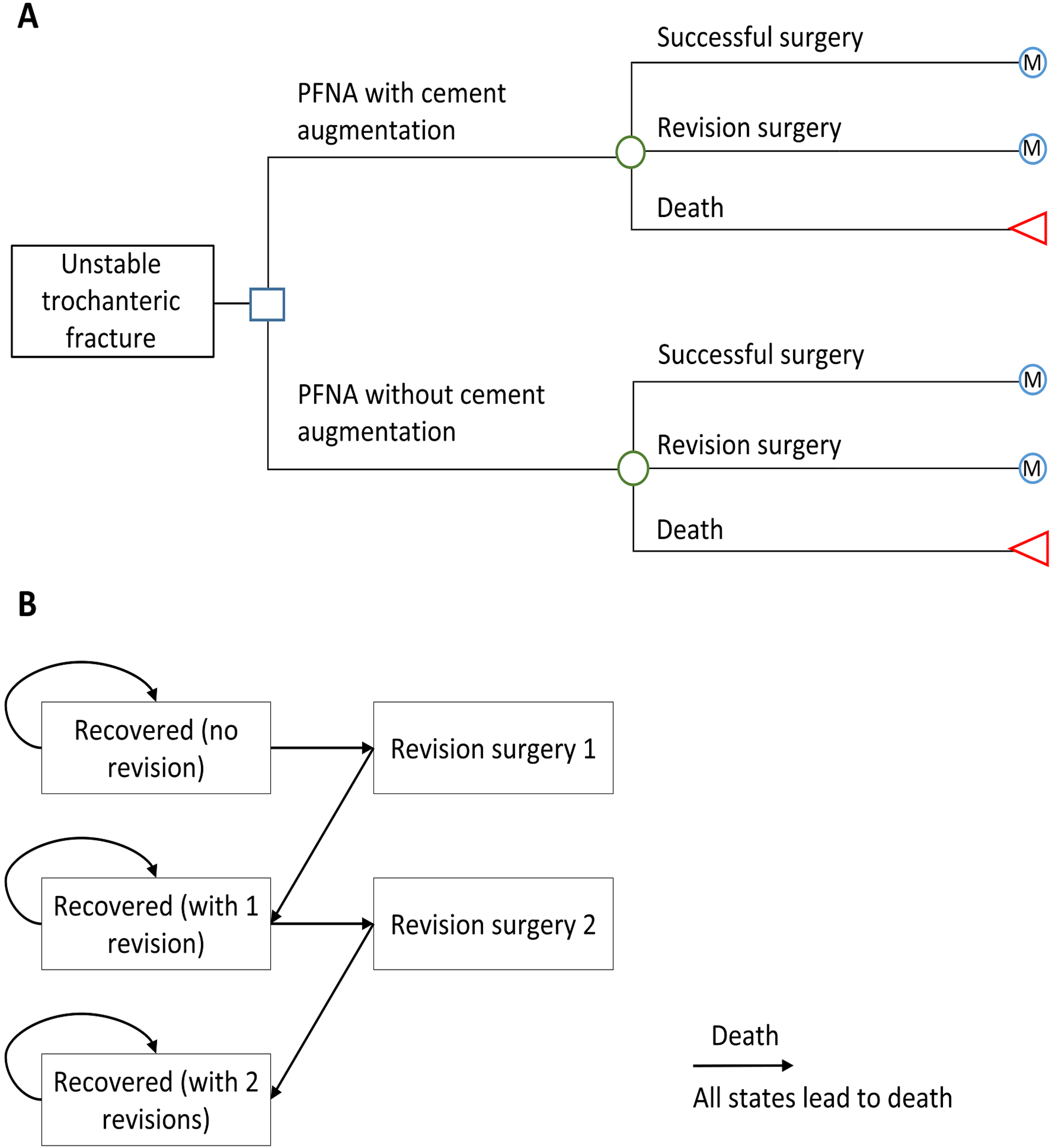
Trochanteric fractures often occur in older patients, including those with osteoporosis, and are associated with high rates of mortality and morbidity. Investigators previously conducted a
Tag: fracture
In this post, Deputy Editor for Social Media Dr. Matt Schmitz discusses the new study by Hong et al. in JBJS: “The Effect of Social
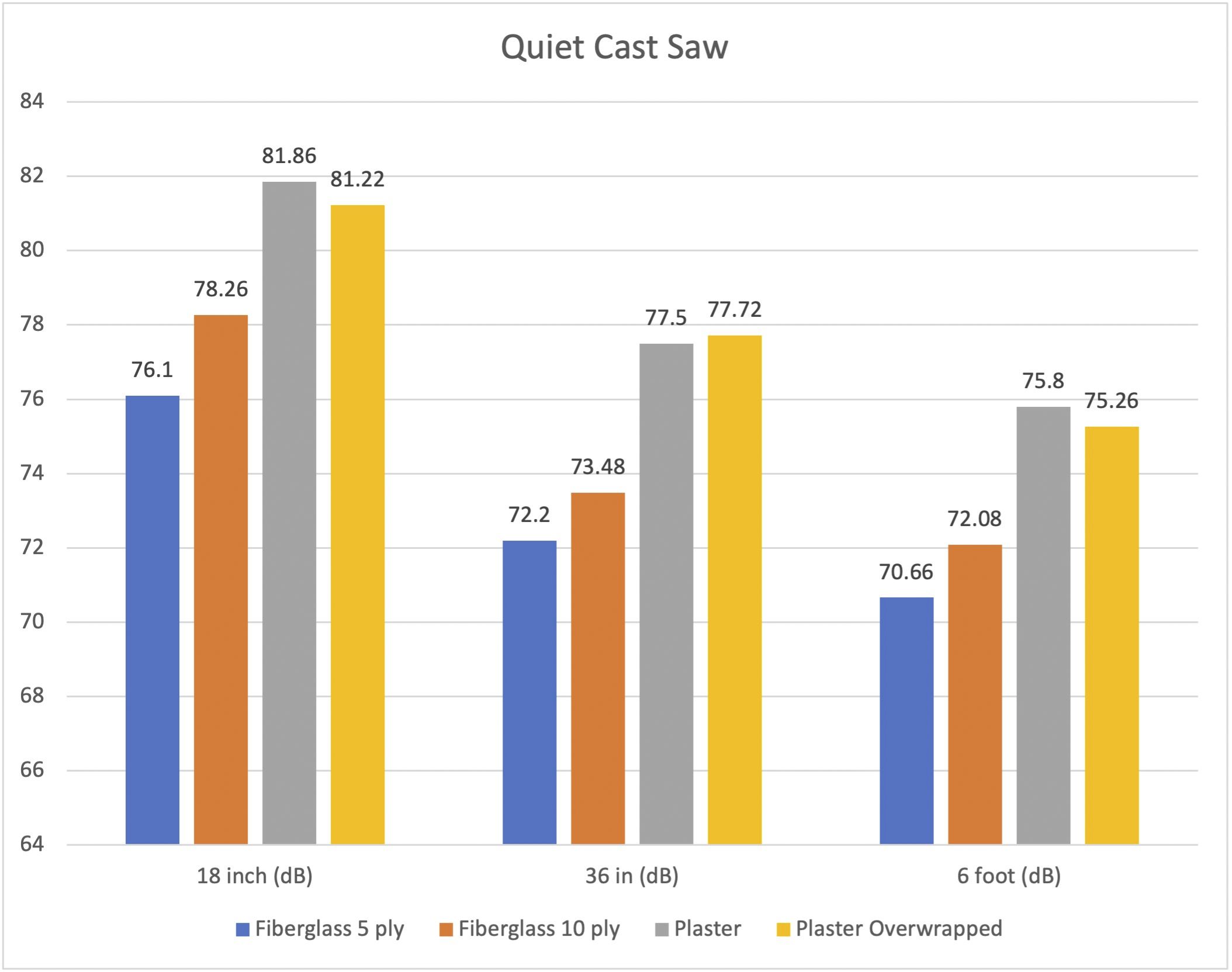
A new study in JBJS examines noise levels during cast removal. In this post, JBJS Editor-in-Chief Dr. Marc Swiontkowski reflects on the importance of research
Recent findings on fracture management and other trauma-related news are presented in the JBJS Guest Editorial “What’s New in Orthopaedic Trauma.” Here, we spotlight the
In a recent report in JBJS, Holler et al. investigated risk factors for delayed management of open tibial fractures in Tanzania. As the authors note,
Approximately 18% of JBJS scientific studies published in 2020 were Level I or II investigations. The number of high-level studies has continued to grow slowly year over year.
Orthopaedic colleagues who live and practice in low-resource areas around the world have clearly voiced that they want support from better-resourced partners. But such efforts
True innovation—improvement way beyond the incremental—is rare in orthopaedics, whether it’s pre- and postoperative management, surgical technique, or prosthetic design. Innovation is even rarer, understandably,
Many animal studies have investigated the impact of nonselective NSAIDs and selective COX-2 inhibitors on fracture healing. Nearly all those experiments focused on chronic drug
As JBJS Editor-in-Chief Marc Swiontkowski, MD observed in a recent editorial, some musculoskeletal health professionals “have been set aside to some degree” during the COVID-19