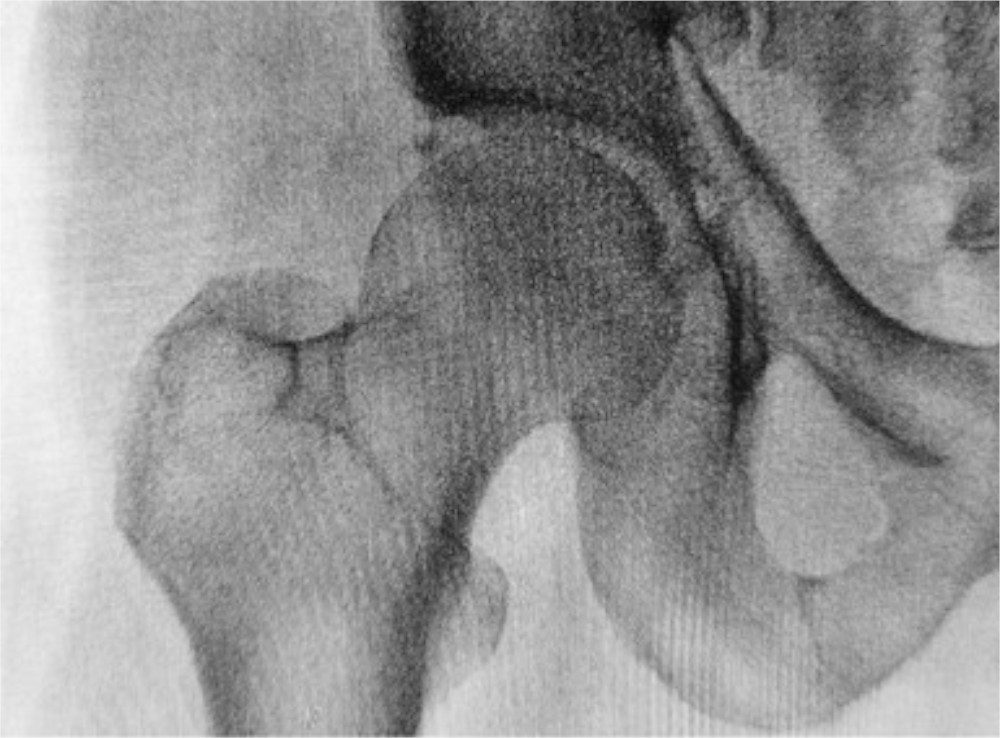
The recent JBJS Guest Editorial “What’s New in Osteoporosis and Fragility Fractures” provides an update on this important area of orthopaedic research. The authors review
Tag: romosozumab
In our ongoing attempt to identify pharmacologic interventions that improve fracture healing, the sclerostin inhibitor romosozumab is a logical candidate, as it has been shown
We have all come to realize that promising results from lab studies or preclinical trials in animal models do not always translate into meaningful clinical