In the current issue of JBJS, Goh et al. report on the association between glucose variability and postoperative complications following aseptic revision total joint arthroplasty
Tag: total joint arthroplasty
Recent studies on periprosthetic joint infection (PJI) and other topics are presented in the JBJS Guest Editorial “What’s New in Musculoskeletal Infection.” Here, we spotlight
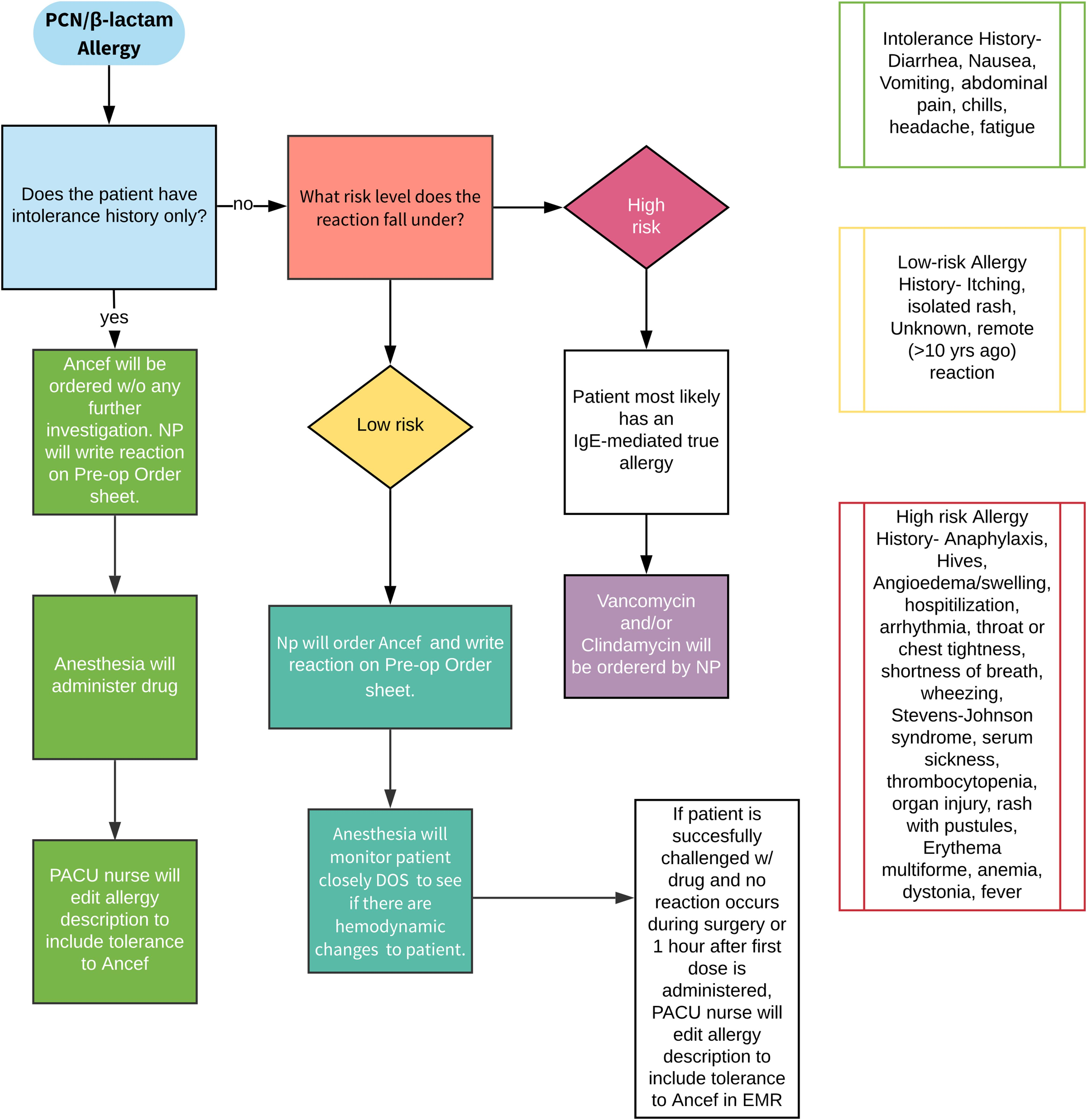
All orthopaedic surgeons must carefully decide on which prophylactic antibiotic to prescribe when a patient or a young patient’s parent reports an allergy to penicillin.
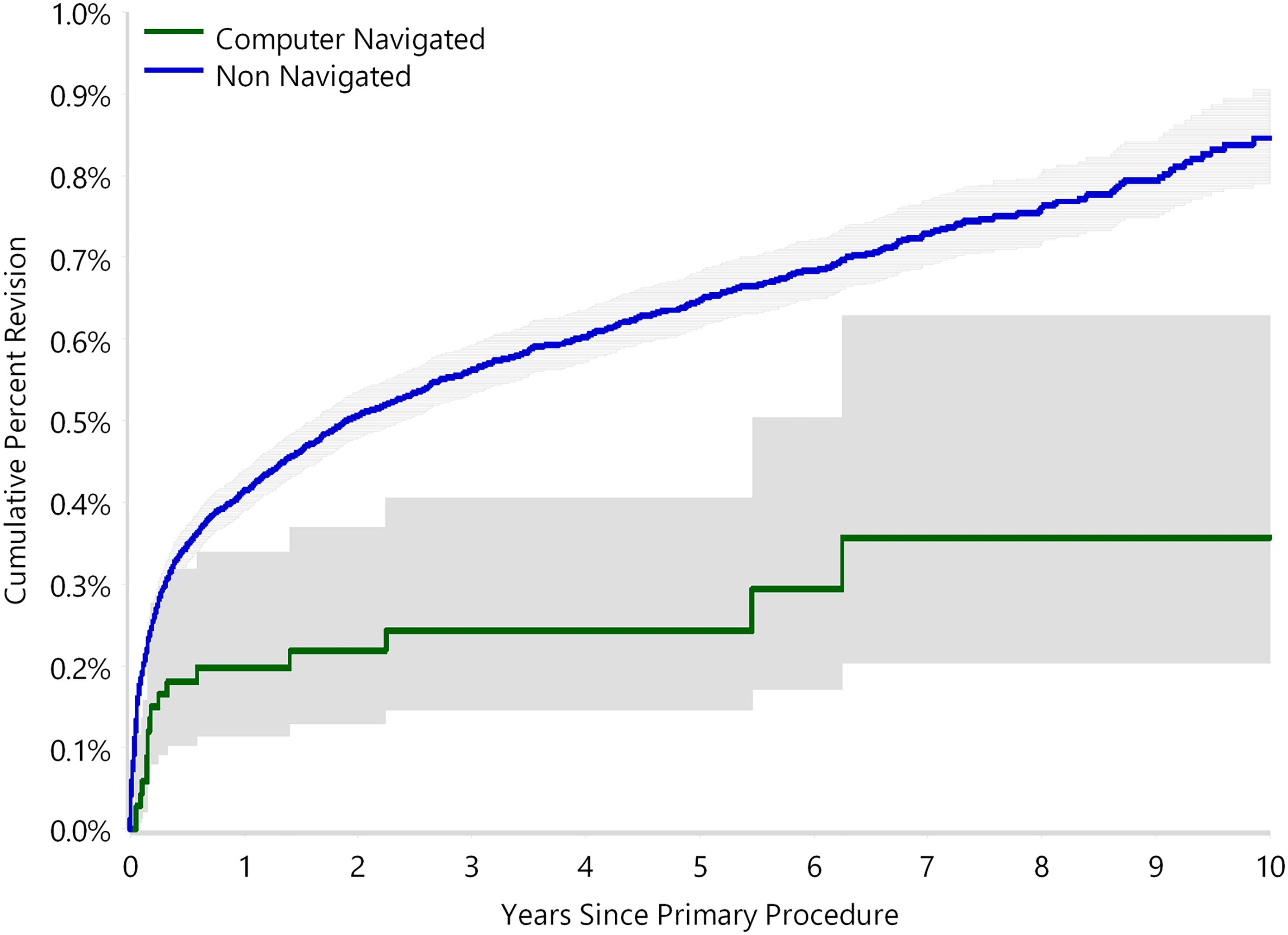
The use of computer navigation in total joint arthroplasty is increasing. Navigation has been employed less in total hip arthroplasty (THA) than in total knee arthroplasty, perhaps because of the increased need for
Co-author Ran Schwarzkopf, MD, MSc discusses the new JBJS study “Tranexamic Acid Is Safe in Patients with a History of Coronary Artery Disease Undergoing Total Joint
Orthopaedic surgeons have long been aware of the role that implant prices play in the total cost of care for arthroplasty procedures, but methodical breakdowns
This post comes from Fred Nelson, MD, an orthopaedic surgeon in the Department of Orthopedics at Henry Ford Hospital and a clinical associate professor at
Over the last 2 decades, research into how various “preexisting conditions” affect the outcomes of orthopaedic interventions has increasingly focused on the impact of mental
Patients with diabetes have an increased risk of postoperative complications following total joint arthroplasty (TJA). Additionally, perioperative hyperglycemia has been identified as a common and
Are you confused and frustrated by Medicare’s Quality-Incentive Programs, such as the Merit Based Incentive Payment System (MIPS), Comprehensive Care for Joint Replacement (CJR) program,