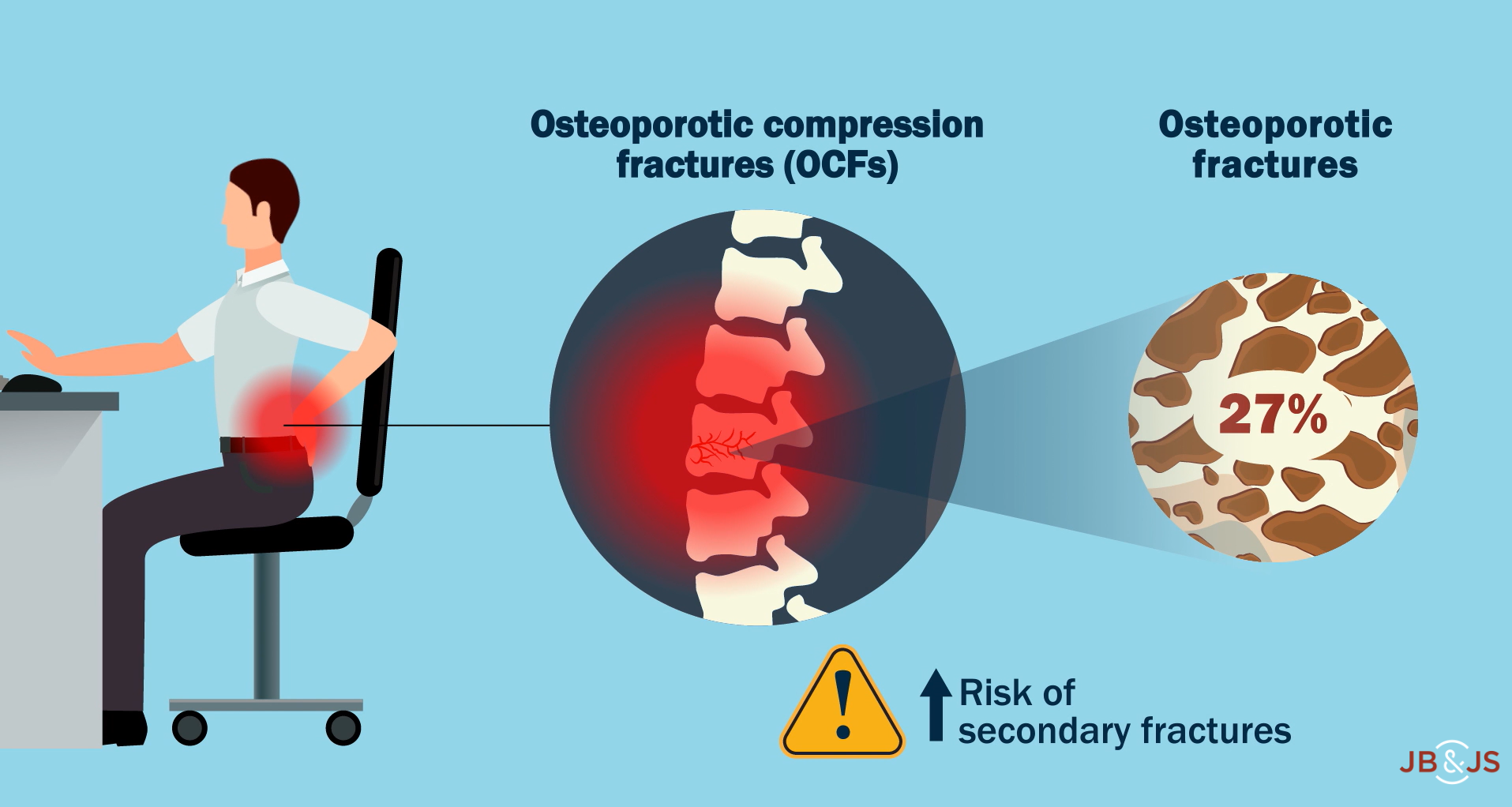
In a new study in JBJS, Mills et al. evaluate whether cement augmentation increases the risk of secondary fracture following a vertebral osteoporotic compression fracture
Search Results for: fragility fracture
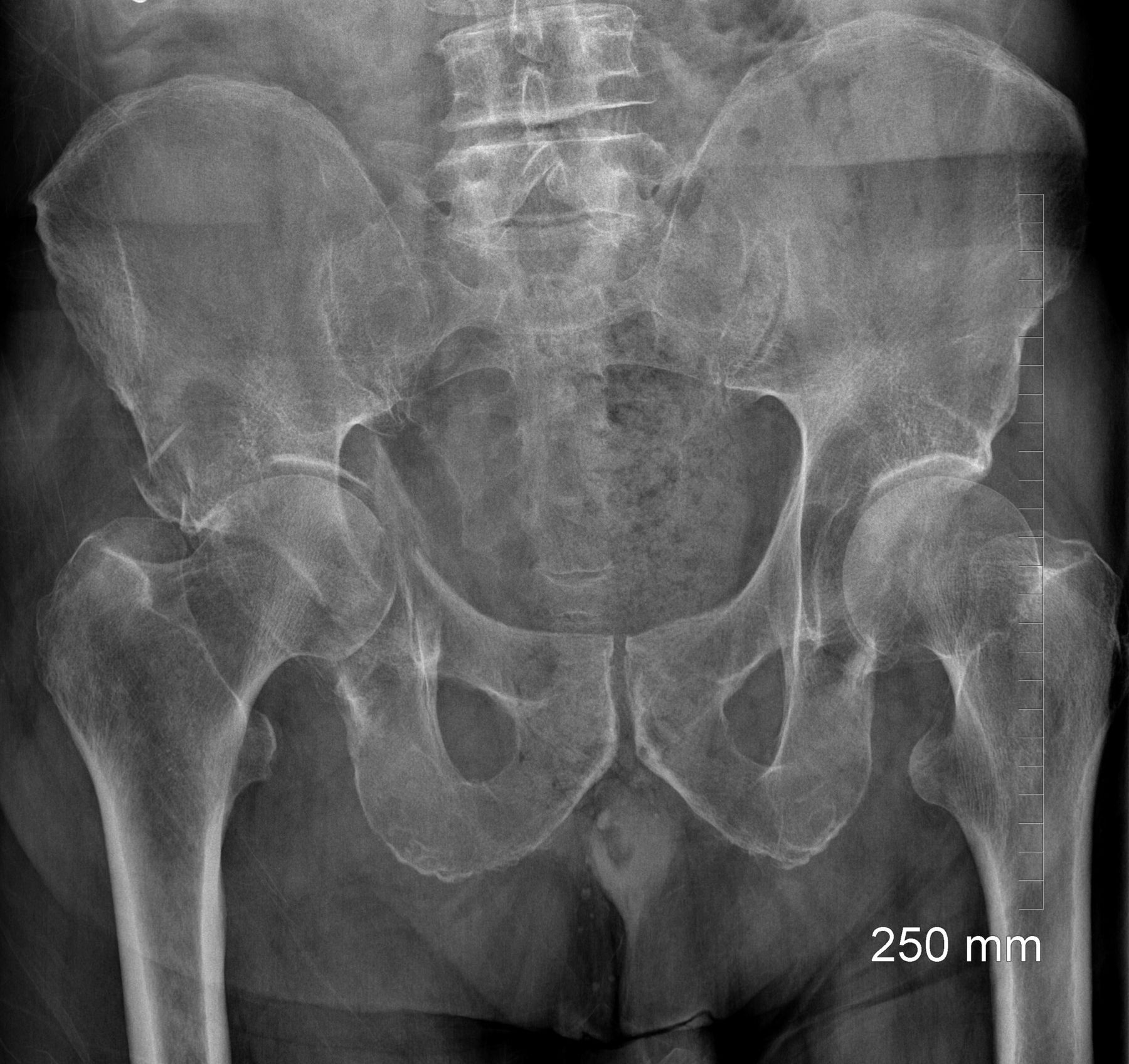
When discussing patient outcomes of hip fractures, we often are speaking of fractures of the proximal part of the femur. But what about the other side of the hip joint, the acetabulum? Among fragility-related injuries, we’re seeing a rise in the incidence of acetabular fractures. And
OrthoBuzz occasionally receives posts from guest bloggers. This guest post comes from James Blair, MD, in response to a recent edition of the OrthoJOE podcast. Geriatric

The Board of Trustees of The Journal of Bone and Joint Surgery is happy to announce that Mohit (Mo) Bhandari, MD, PhD, FRCSC, has been

Noteworthy findings regarding topics such as platelet-rich plasma injection, botulinum toxin injection, and microinvasive trigger-finger release, among others, are presented in the new JBJS Guest
Recent findings in rotator cuff repair, shoulder arthroplasty, and fracture management are among the topics of interest in the new JBJS Guest Editorial What’s New
Periprosthetic joint infection, venous thromboembolism prevention, and implant cost-utility are among the focuses of the new JBJS Guest Editorial What’s New in Hip Surgery. Here,
Osteoporosis is the major contributor to the increasing incidence of fragility fractures associated with low-energy falls. The other contributor is the populous baby-boomer generation that
Botulinum toxin treatment of plantar fasciitis, clinical applications of point-of-care ultrasound, and other key topics are presented in the new JBJS Guest Editorial “What’s New
Implant design, socioeconomic inequality, and opioid prescriptions are some of the topics of the recent studies summarized in the JBJS Guest Editorial “What’s New in