In severe musculoskeletal trauma, studies suggest that patients may have better long-term outcomes when interventions in the early recovery period support psychosocial health needs. A
Tag: JBJS
Rotator cuff repair, shoulder arthroplasty, shoulder instability, and the treatment of elbow injuries are among the topics of recent studies reviewed in the new JBJS
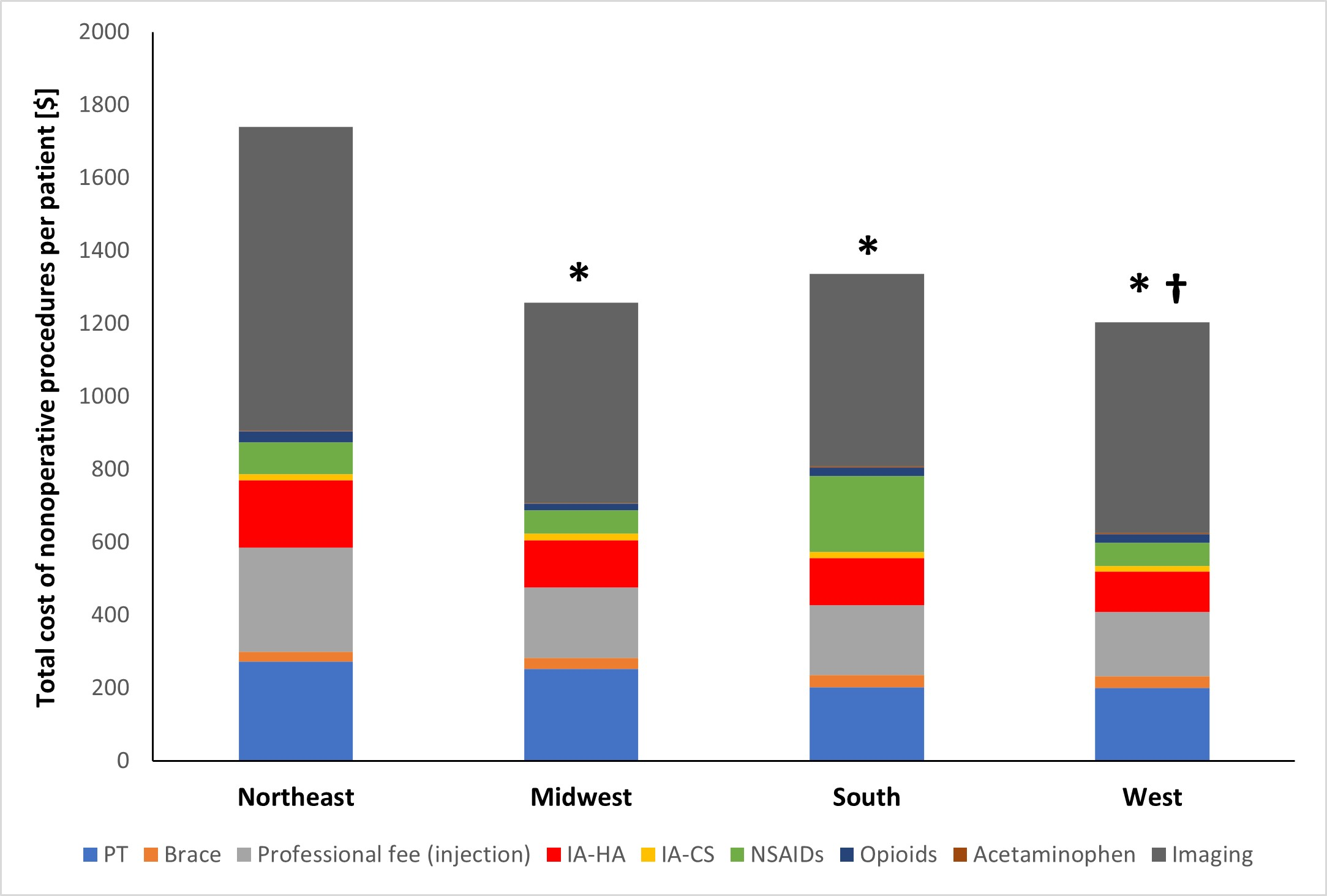
A new JBJS study reviews costs associated with nonoperative management of osteoarthritis in the 1-year period leading up to total knee arthroplasty (TKA). JBJS Deputy

A recent article spotlights what appears to be a high rate of “honorary authorship” in scientific publishing. The term refers to the controversial practice of
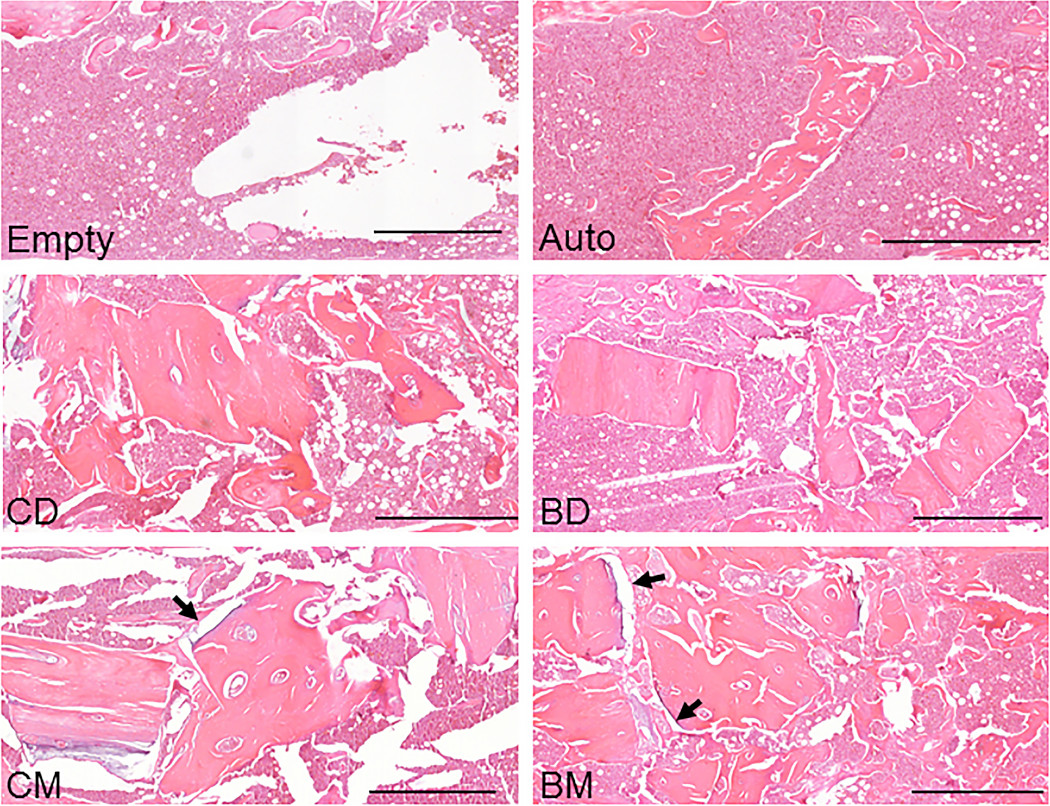
The findings of a new animal study suggest that bisphosphonate treatment in donors may indeed be relevant when mineralized allografts are used in orthopaedic procedures.
Implant design, socioeconomic inequality, and opioid prescriptions are some of the topics of the recent studies summarized in the JBJS Guest Editorial “What’s New in

The importance of LGBTQ+ representation in orthopaedic surgery and the formation of Pride Ortho are the focus of a current JBJS Orthopaedic Forum article. “New

OrthoBuzz readers who’d like to learn more about the Major Extremity Trauma and Rehabilitation Consortium (METRC) are encouraged to check out the recent episode of
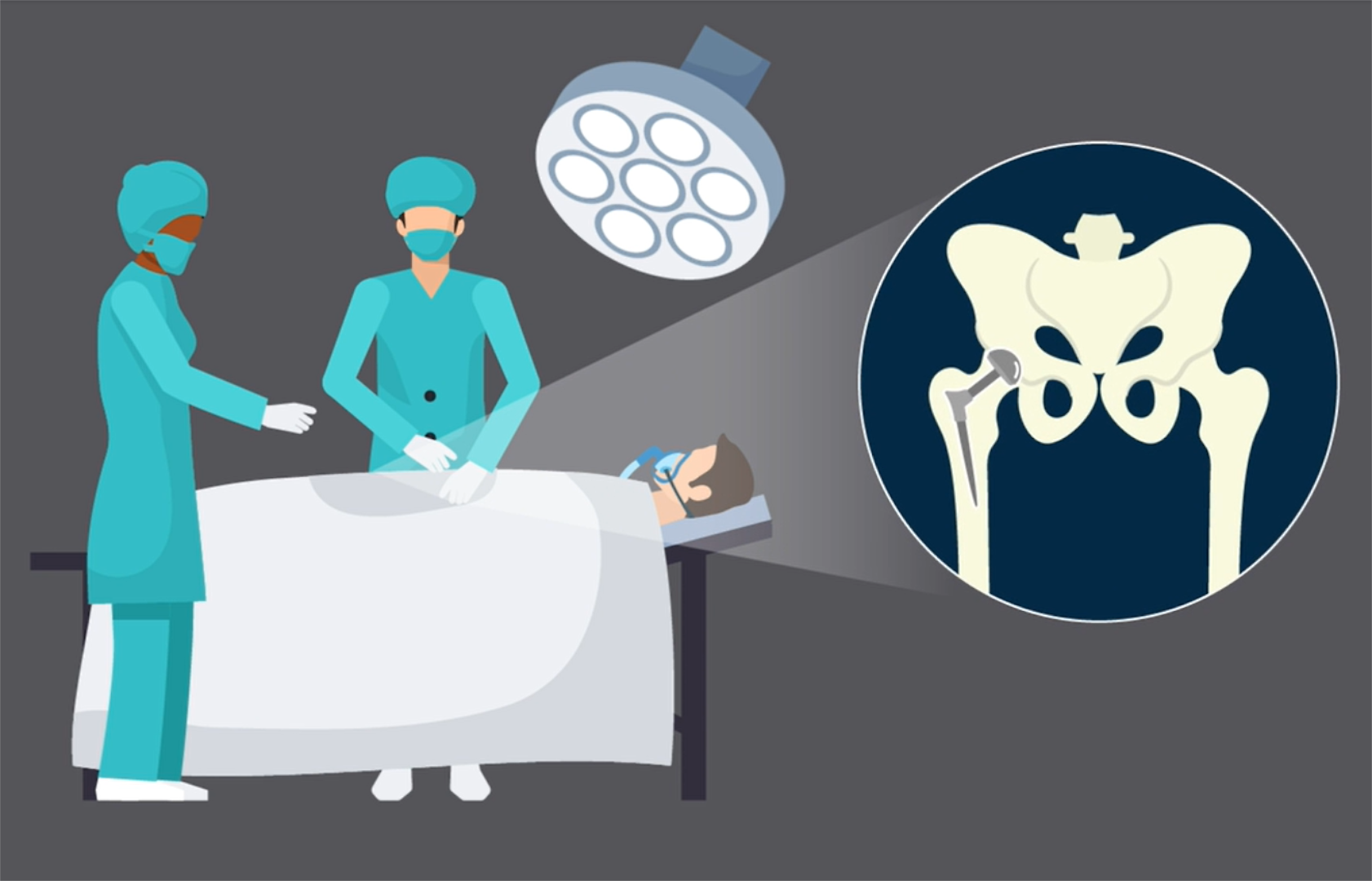
In the current issue of JBJS, Goh et al. report on the association between glucose variability and postoperative complications following aseptic revision total joint arthroplasty

This week marks Peer Review Week, an annual event to celebrate the value of peer review by those in scholarly communications. As readers know, JBJS

