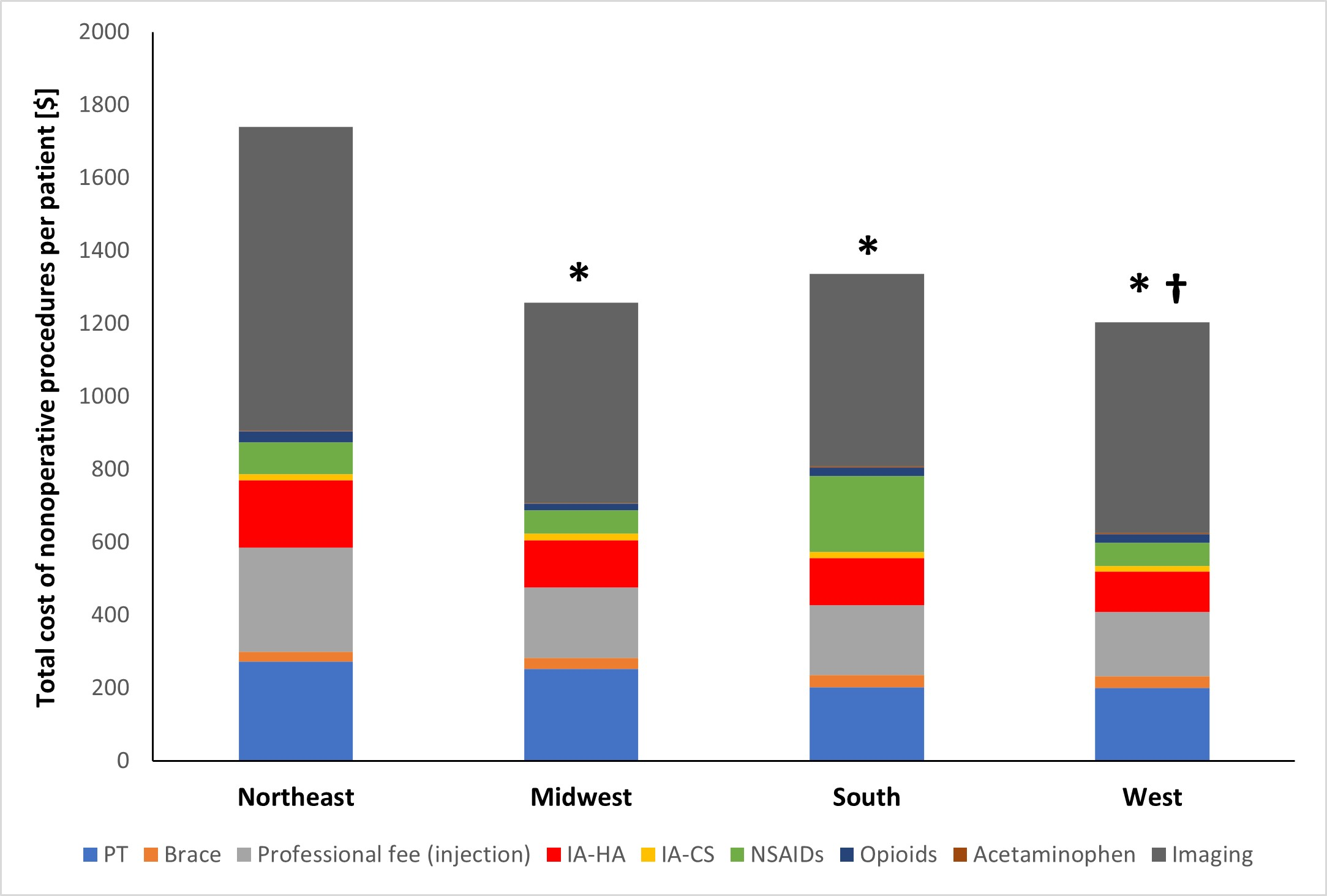
A new JBJS study reviews costs associated with nonoperative management of osteoarthritis in the 1-year period leading up to total knee arthroplasty (TKA). JBJS Deputy
Tag: Osteoarthritis
Implant design, socioeconomic inequality, and opioid prescriptions are some of the topics of the recent studies summarized in the JBJS Guest Editorial “What’s New in
Intra-articular hyaluronic acid injections, also known as viscosupplementation, are a common treatment option for many patients with end-stage osteoarthritis of the knee. However, the effectiveness

JBJS Journal of Orthopaedics for Physician Assistants (JOPA) continues the tradition of recognizing outstanding papers. A total of 4 awards are given for the best
A new study evaluates trends in knee arthroscopy. Have evidence-based recommendations influenced real-world practice? This post comes from research resident Derek T. Schloemann, MD,

Co-author Rachel M. Frank, MD summarizes the 5 most compelling findings from among the studies highlighted in the new “What’s New in Sports Medicine” in JBJS.
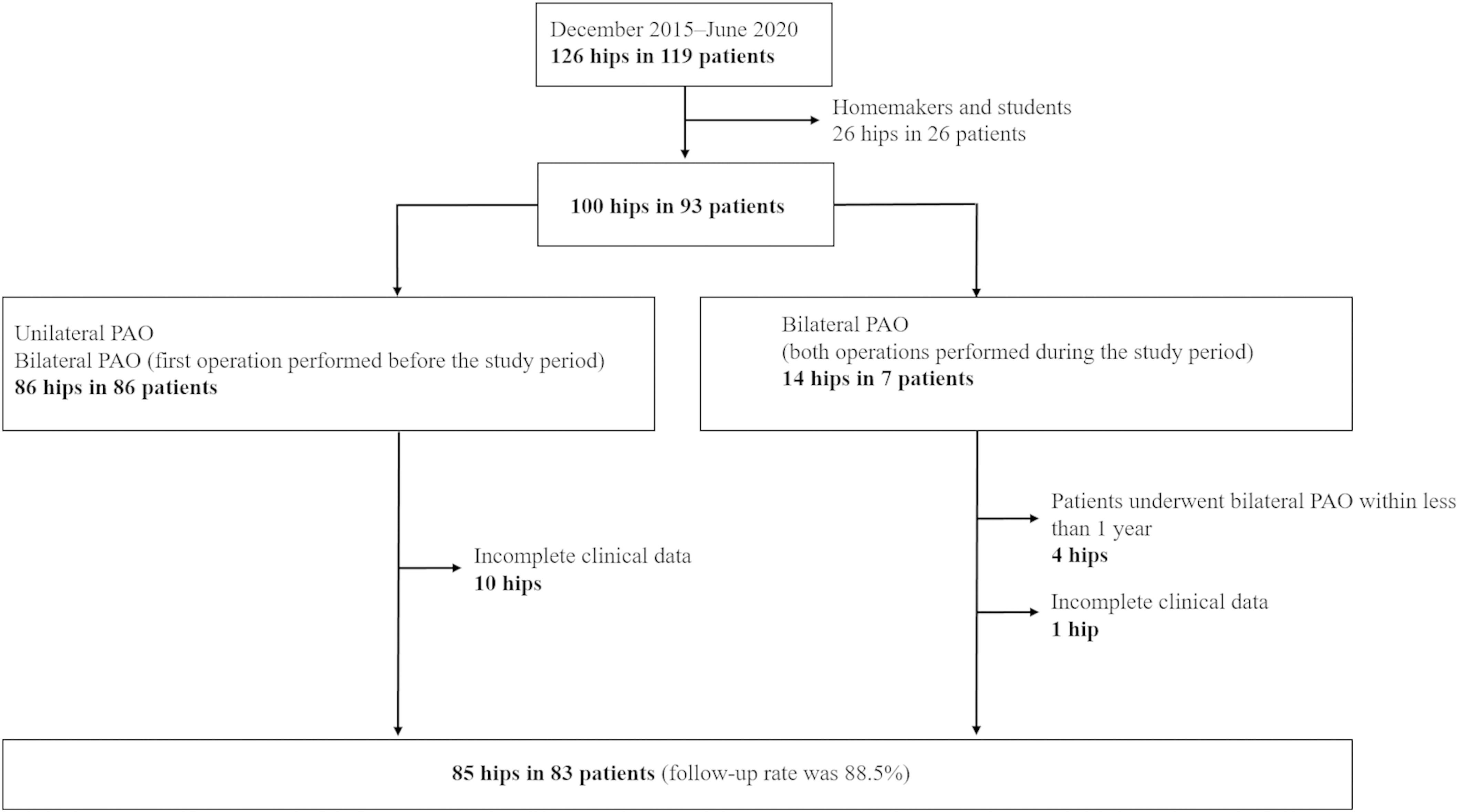
A periacetabular osteotomy (PAO) is a surgical treatment for hip dysplasia that has been shown in adult patients to delay the onset of hip arthritis
This guest post comes from Jaime Bellamy, DO in response to a recent randomized clinical trial reported in JAMA Network Open and featured in MedPage
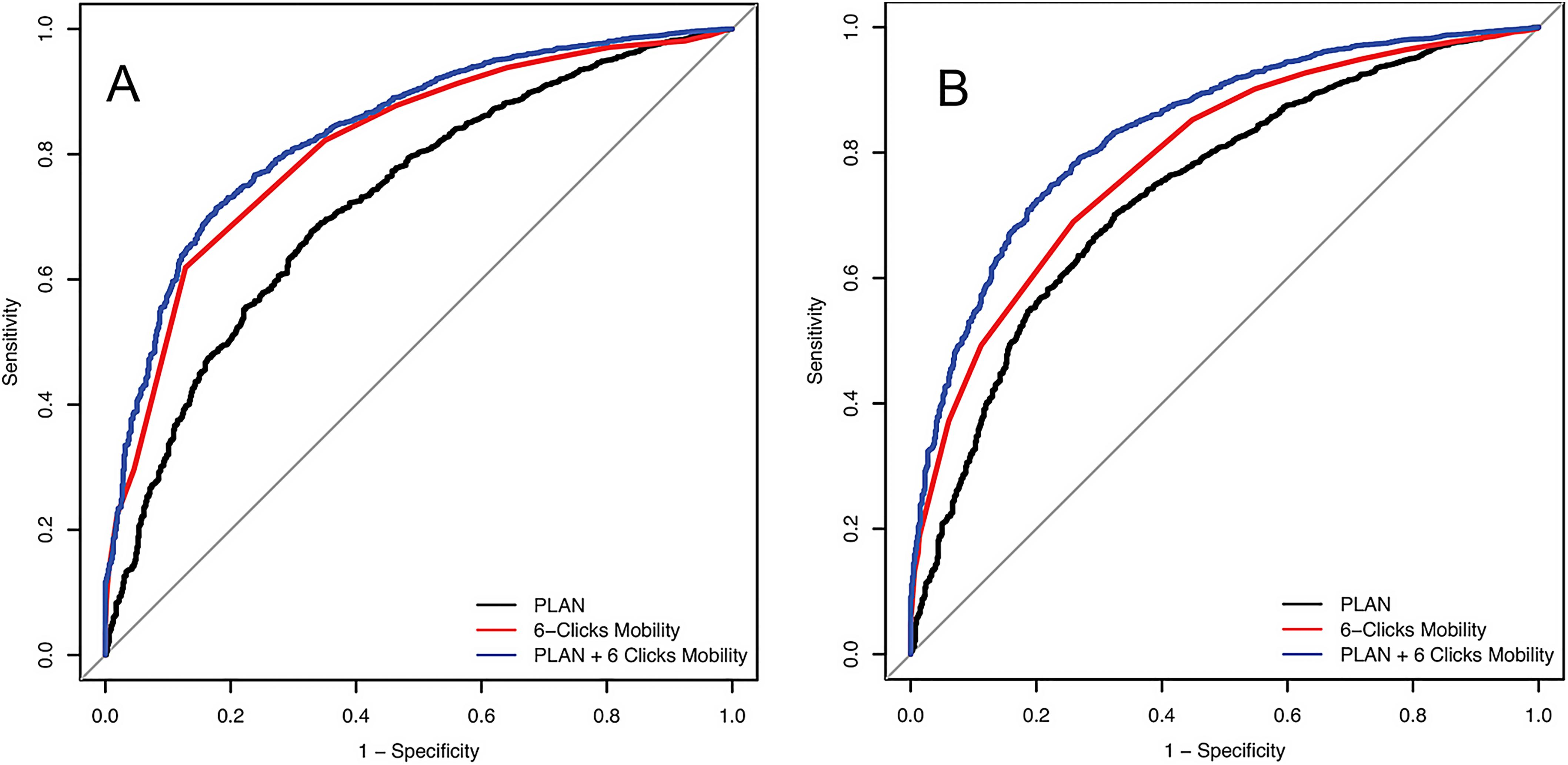
Improving Our Ability to Predict Discharge Disposition After Primary Total Hip and Knee Arthroplasty
The majority of patients who undergo primary total hip arthroplasty (THA) and total knee arthroplasty (TKA) are discharged to home postoperatively, but for some patients,
This guest post comes from David Vizurraga, MD in response to a study in JAMA investigating platelet-rich plasma vs. placebo in the treatment of knee