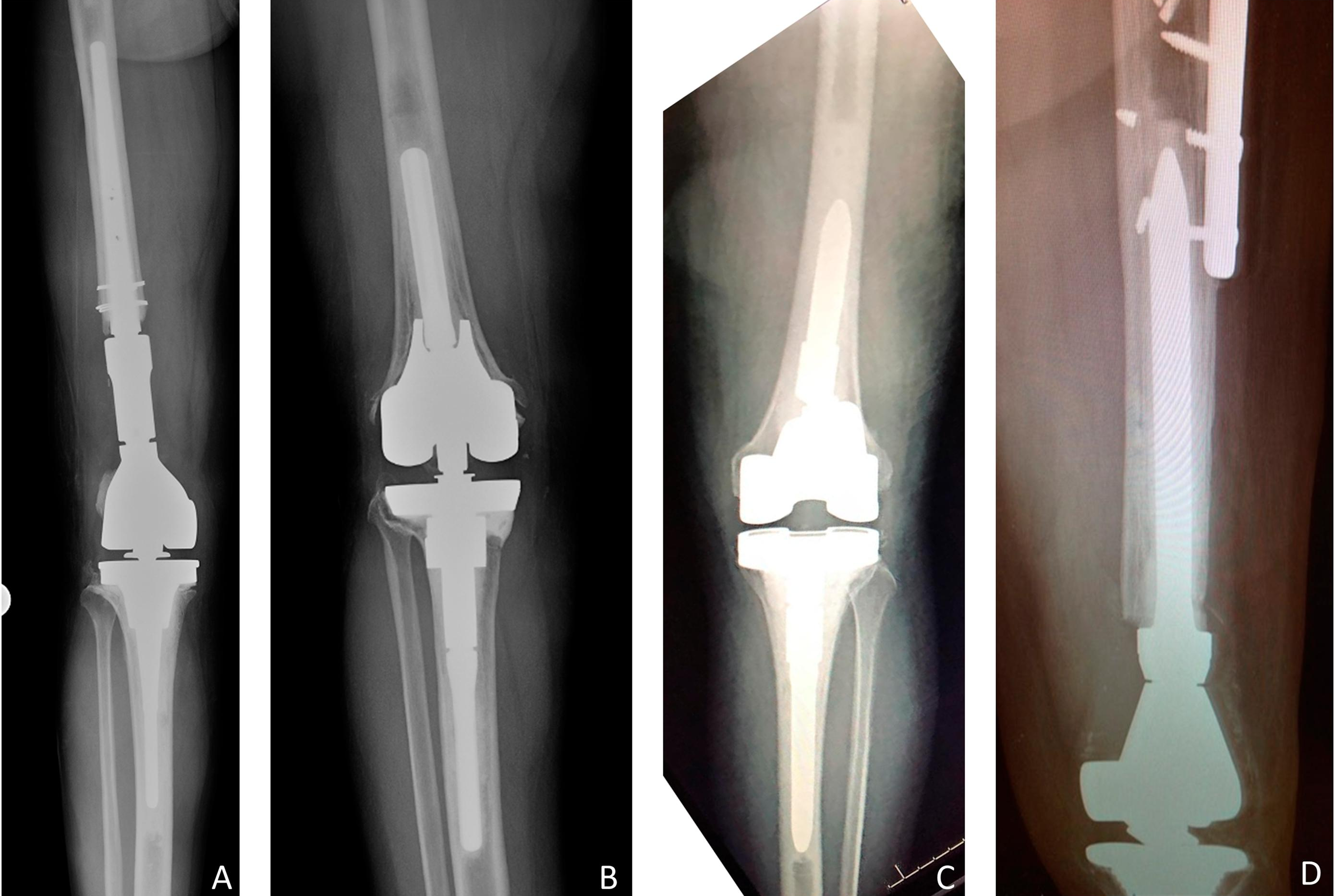
Recent studies on periprosthetic joint infection (PJI) and other topics are presented in the JBJS Guest Editorial “What’s New in Musculoskeletal Infection.” Here, we spotlight
Tag: PJI
Every month, JBJS publishes a review of the most pertinent and impactful studies from the orthopaedic literature during the previous year in 14 subspecialty areas. This month, co-author
Periprosthetic joint infection (PJI) remains a challenging complication in orthopaedics. These infections are often related to low-virulence organisms, and the search for reliable diagnostic tests continues to be paramount. Joint aspiration has been a starting point for
The incorporation of antibiotics within polymethylmethacrylate (PMMA) has been widely used over recent decades for managing infection following skeletal trauma. Early research helped to
Every month, JBJS publishes a review of the most pertinent and impactful studies published in the orthopaedic literature during the previous year in 14 subspecialties. Click
Among >100,000 total hip arthroplasty (THA) patients ≥55 years of age whose data resides in a Canadian arthroplasty database, the 15-year cumulative incidence of periprosthetic
Every month, JBJS publishes a review of the most pertinent and impactful studies published in the orthopaedic literature during the previous year in 13 subspecialties. Click here for
Prior to performing a primary total joint arthroplasty, patient optimization is both possible and recommended. However, when a patient with a periprosthetic joint infection (PJI)
Despite a bevy of research and intense clinical focus, definitively diagnosing periprosthetic joint infections (PJIs) remains a major challenge in many patients. There is no
The US FDA has approved the Synovasure Alpha Defensin Lateral Flow Test Kit for helping detect periprosthetic joint infection (PJI) in the synovial fluid of